Abstract
Pentraxin-3 has been reported as a promising biomarker of pre-eclampsia and its severity; however, available studies have small sample sizes, and analyses are not always adjusted for confounders. The aim of this study is to establish the strength of the association between maternal Pentraxin-3 level and pre-eclampsia or HELLP syndrome. It was a case-control study. Women with pre-eclampsia or HELLP syndrome were defined as cases, and women with healthy pregnancies at term (>37 weeks) were classified as controls. Plasma concentrations of Pentraxin-3 were determined at the time of delivery by quantitative enzyme immunoassay. Associations between Pentraxin-3 and pre-eclampsia and HELLP syndrome were assessed by multinomial logistic regression. Subsidiary analysis for the time of disease onset was also carried out. Odds ratios and 95% confidence intervals are reported. A total of 1024 pregnant women were included (461 controls, 368 pre-eclampsia, 195 HELLP). A positive log-linear relationship was found between the top pentraxin-3 quintile and HELLP syndrome. After adjustment for confounders (maternal age, ethnicity, socioeconomic position, date and place of recruitment, family history of pre-eclampsia, smoking, body mass index at beginning of pregnancy, gestational age and multiple pregnancy), the strength of the association was higher for HELLP syndrome [OR 1.13 (95% CI 1.08; 1.18)] than for pre-eclampsia [OR 1.03 (95% CI 1.03; 1.10)]. No difference according to time of onset or pentraxin-3 level was found. In summary, pentraxin-3 level was associated with pre-eclampsia, but it was more strongly associated with HELLP syndrome. Longitudinal studies with a lower probability of residual confounding are necessary to improve our knowledge about the role of pentraxin-3 in pre-eclampsia.
Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy, such as pre-eclampsia, represent an important cause of maternal and perinatal morbidity and mortality worldwide, especially in low- and middle-income countries (LMICs) [1]. For 2006, the World Health Organization (WHO) estimated that these disorders were responsible for 16.1% (95% CI 6.7–24.3%) of maternal deaths in developed countries (44). It was higher for countries in Latin America and the Caribbean (25.7%, 95% CI 7.9–52.4%) [2]. The incidence of preeclampsia is ~5–10% of all pregnancies [3, 4], and despite its high burden, its complete pathogenesis is not fully understood. Two main findings have been identified at the beginning of pregnancy: disruption of uterine vascular remodeling and an anti-angiogenic response [5]. The final result of all pathophysiological processes is systemic endothelial dysfunction, which has been associated with several vasoconstrictors, oxidative stress and inflammatory biomarkers in maternal circulation [4, 6]. However, precise inflammatory biomarkers that are causally related to pre-eclampsia remain to be identified.
Pentraxin-3 (PTX3) is an acute-phase protein. It is produced and released by several cell types in response to primary inflammatory stimuli, in particular by mononuclear phagocytes, dendritic cells, fibroblasts, vascular endothelial and smooth muscle cells [7,8,9]. PTX3 is elevated in endotoxic shock, sepsis, and vascular disorders, such as small vessel vasculitis and acute myocardial infarction, and correlates with outcome or disease activity [10,11,12]. In the nonpregnant state, PTX3 is expressed in response to cytokines regulating inflammation and apoptosis [7]. On the other hand, PTX3 has been proposed as a novel biomarker predicting placental failure. Zhou et al. [13] reported that the expression of PTX3 and TNF-α in placental tissues and maternal sera was significantly increased in pre-eclamptic patients, as well as in those with intrauterine growth restriction (IUGR). PTX3 is mainly expressed in villous stroma, decidual cells and terminal villi, and TNF-α is mostly localized in trophoblasts, vascular endothelial cells, decidual cells and in the stroma of the stem villi, which support the possibility that PTX3 is involved in the pathogenesis of pre-eclampsia [13]. In early pregnancy, PTX3 may play an important role in successful implantation [14]. Likewise, two studies in the Italian population (<50 pre-eclamptic patients) have reported that the plasma level of PTX3, measured during prenatal visits or at the moment of disease diagnosis, was higher in pre-eclamptic than in normal pregnancies; however, these studies did not adjust for confounders in their association analysis [15, 16]. More recently, PTX3 levels assessed since the beginning of pregnancy have shown a rising trend in both healthy and pre-eclamptic pregnancies; nonetheless, PTX3 levels during the first and second trimesters were significantly higher in pre-eclamptic pregnancies than in healthy pregnancies [17]. HELLP syndrome is a serious complication of pregnancy characterized by hemolysis (H), elevated liver (EL) enzymes, and low platelet (LP) count, occurring in 0.2–0.6% of all pregnancies and in 10–20% of cases with severe pre-eclampsia [18, 19]. There is still debate on whether HELLP syndrome must be considered a severe form of pre-eclampsia or a separate disease. In general, it is agreed to be a disease induced by the placenta, like pre-eclampsia itself, but with a more acute and predominant inflammatory process typically targeting the liver and with a greater activation of the coagulation system [20]. In this way, only one study so far has shown an association between PTX3 level and disease severity, including HELLP syndrome [21]. The aim of this study was to assess the strength of the association of maternal plasma PTX3 level with pre-eclampsia and its severity in a large, multicenter, case-control study using multivariate analysis with adjustment for possible confounders.
Methods
Study design and participants
This is a case-control study that is part of the GenPE study (Genetics and Pre-eclampsia). GenPE is a large, multicenter, case-control study conducted between December 2000 and February 2012. Pregnant women were recruited at the time of delivery in eight Colombian cities (www.genpe.org) [22]. Pre-eclampsia was defined as blood pressure ≥140/90 mmHg on two separate occasions within a 24-h period and proteinuria ≥300 mg in 24 h or ≥1+ dipstick reading in a random urine sample with no evidence of urinary tract infection after the 20th week of pregnancy [23]. A control was defined as a normotensive woman with an uneventful pregnancy delivering by labor or cesarean section at term (37–42 weeks). At least one control was recruited from the same center that provided the case within 1 week (aiming for 24 h) of the case identification. In order to improve homogeneity of phenotypes, women with a prior history of autoimmune, metabolic (including diabetes or gestational diabetes), renal, or cardiac (including chronic hypertension) diseases were excluded from the study. HELLP syndrome was diagnosed when a woman of any age and parity had PE, a complete or incomplete pattern of hemolysis, elevated liver enzymes and thrombocytopenia as defined by Sibai [24]. Pre-eclampsia sub-phenotypes were defined according to the time of onset: early pre-eclampsia for presentation before 34 weeks, intermediate pre-eclampsia for presentation from 34 weeks to less than 37 weeks, and late pre-eclampsia for presentation from 37 weeks of gestation onwards.
Data collection
A verbal interview with a structured questionnaire was conducted by trained personnel to ascertain demographic and clinical data such as maternal age, race, socioeconomic position (ranging from 0 to 6, with 0 being the most deprived and 6 being the most affluent, and low socioeconomic status defined as below 3) [25], smoking (defined as having smoked any quantity for any time of any tobacco product at any moment of gestation) and infection during pregnancy (e.g., vaginosis, urinary tract infections, sexually transmitted diseases and others), family history of pre-eclampsia, gestational age at recruitment, blood pressure, proteinuria level, and medical data of the offspring (height, weight, Apgar). Blood pressure was measured in the right arm after a five-minute period of rest in a seated position using a mercury sphygmomanometers or electronic devices calibrated against a mercury standard [26]. Mean arterial pressure (MAP) was estimated as diastolic blood pressure +0.412 × (systolic blood pressure − diastolic blood pressure), as suggested by Papaioannou et al. [27]. PTX3 levels were measured in 1024 unrelated young pregnant women recruited at the time of delivery from seven hospitals in five Colombian cities between January 2001 and September 2009. This sample size could detect a 1.5 odds ratio in association analysis considering 20% exposure in controls, with 80% power and 95% confidence. All participants signed a written informed consent form at recruitment and prior to inclusion in the study.
Blood sampling and PTX3 measurement
Blood samples were drawn at recruitment (at delivery) from the antecubital vein using EDTA-treated tubes (Becton Dickinson, USA). Samples were centrifuged for 10 min. The obtained plasma was transferred to vials in fractions of 300 µL and stored at −80 °C until the PTX3 assay was performed. Plasma concentrations of PTX3 were determined by the quantitative sandwich enzyme immunoassay (ELISA) technique. A duplicate analysis was done for all samples. The assay was repeated when the difference in absorbance was greater than 0.087. When standardizing our process, an initial standard curve was made with doubling dilutions ranging from 75 pg/ml to 2.4 ng/ml, but the samples in our patients showed values of more than twice the absorbance of the highest point in this standard curve. For this reason, it was necessary to make a series of curves to cover higher concentrations. In the end, the standard curve was established at a range of 2.4–19.2 ng/ml, maintaining an R2 of 0.986 with an intercept of 0.032 OD. The standardization was performed by two different persons to overcome the inherent operator errors. Absorbance was read at 450 nm in an ELISA automatic reader (Microplate Absorbance Reader, software Microplate Manager 6, Bio-Rad, USA). The concentrations of PTX3 were determined using Microplate Manager 6 software from Bio-Rad; the minimum value detected was 0.030 ng/ml. PTX3 measurement was conducted by a laboratory technician blinded to the case-control status. In addition, the plasma samples of cases and controls were randomly distributed across plates, and the codes assigned to the samples were consecutive, thus making it impossible to discriminate between cases and controls based only on the codes. Additional information on PTX3 measurement is described in the Supplementary material.
Statistical analysis
Continuous variables are described as mean and standard deviation (SD); nonnormally distributed variables are reported as median with interquartile range. Categorical variables are described as counts and proportions. To evaluate differences between groups, the χ2 test, Fisher’s exact test, one-way ANOVA or the Kruskal–Wallis test was used, as appropriate. Eight samples had PTX3 levels four SD above the mean and were considered outliers; this affected the functional relationship between exposure and outcome (Supplementary Material – Fig. 1), and they were removed from further association analysis after assessment of multinomial regression coefficients (Supplementary Material Table 1).
Association analysis
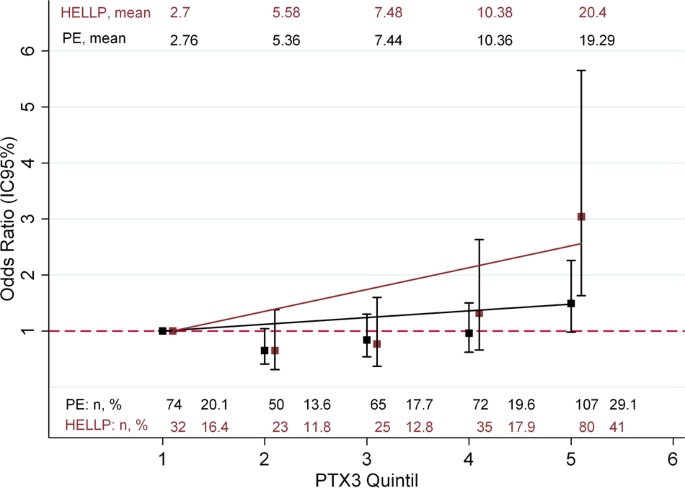
A multinomial logistic regression analysis was carried out to assess the log-linear relationships between PTX3 and pre-eclampsia and HELLP syndrome. PTX3 was categorized into quintiles according to its distribution in the control group [<4405 ng/ml (Q1), 4405–6321 ng/ml (Q2), 6322–8678 ng/ml (Q3), 8679–12,135 ng/ml (Q4), >12,135 ng/ml (Q5)], using the bottom quartile as a reference. A test for a linear trend across quintiles was performed using a multinomial regression model adjusted for maternal age. To establish the strength of the association between PTX3 level (as a continuous variable) and pre-eclampsia and HELLP syndrome, a multinomial logistic regression analysis was carried out. In addition, a subsidiary analysis according to the time of onset was performed using multinomial regression analysis. Analyses progressed from unadjusted to adjusted, after an a priori consideration of the following variables as potential confounders: maternal age, ethnicity, socioeconomic position, date and place of recruitment, history of pre-eclampsia in their mother’s or sister’s pregnancies, smoking, body mass index at the beginning of pregnancy, gestational age and multiple pregnancy. For model comparison, the likelihood ratio test was used (Supplementary Material Table 2). Odds ratios and 95% confidence intervals (CIs) are reported. Data analysis was performed with Stata 13 (StataCorp, USA). This investigation conformed to the principles outlined in the Declaration of Helsinki. All participants signed an informed consent document approved by the Ethical Review Board from Universidad Autónoma de Bucaramanga, Colombia (Research Ethics Committee. Act No. 0037/2007. August 29, 2007). The founding source was not involved in the collection, data analysis or submission process of the manuscript.
Results
Characteristics of the study population
A total of 1024 women were included in the study (461 controls, 368 with pre-eclampsia, 195 with HELLP syndrome). The characteristics of the women and their newborns are displayed in Table 1. Most women were of mixed ethnic ascendency and from a low socioeconomic position. Those with HELLP syndrome were older and had higher prepregnancy weight and earlier gestational age than women with pre-eclampsia or normal pregnancy. In addition, women with pre-eclampsia or HELLP had higher blood pressure and delivered newborns with lower weight.
Association analysis
A significant positive log-linear relationship was found only for the top quintile of PTX3 and HELLP syndrome (Fig. 1); the p value for the linear trend across quintiles was <0.001 for both stages of disease severity. After adjustment for all potential confounders, multinomial logistic regression showed that per unit increase (1 ng/ml) in PTX3 level, the odds of pre-eclampsia and HELLP syndrome increased in this population (Table 2). Maternal and gestational age were the most influential confounders on adjustment (Supplementary Material). Subsidiary analysis according to the time of onset is shown in Fig. 2. PTX3 level showed an OR of 1.07 (95% CI 1.01; 1.14) for early pre-eclampsia, an OR of 1.01 (95% CI 0.96; 1.07) for intermediate pre-eclampsia and an OR of 1.07 (95% CI 1.03; 1.11) for late pre-eclampsia. However, the confidence intervals overlapped at different times of disease onset, as well as for HELLP syndrome [early: OR 1.12 (95% CI 1.06; 1.19), intermediate: OR 1.16 (95% CI 1.10; 1.24) and late: OR 1.13 (95% CI 1.07; 1.20)].
Association between PTX3 level and time of pre-eclampsia onset in the pre-eclampsia and HELLP groups. OR indicates the change in the odds of having early, intermediate or late pre-eclampsia (a) or HELLP syndrome (b) conferred by each one-unit increase in PTX3 level. Adjusted for maternal age, ethnicity, recruitment place and date, low socioeconomic position, familial history of pre-eclampsia, smoking, BMI and multiple pregnancy. Gestational age was not included in the model because this variable defined the subphenotype assessed. OR from multinomial logistic regression for pre-eclampsia (All PE) and HELLP Syndrome (All) are included only for comparison purposes. Odds ratios are reported on a logarithmic scale
Discussion
In this case-control study, we found significant associations of the maternal plasma level of PTX3 with pre-eclampsia and, especially, with HELLP syndrome. To our knowledge, this is the largest report (including ~10 times more pregnant women than previous studies) investigating PTX3 circulating levels in the spectrum of severity of pre-eclampsia.
PTX3 was initially described as an early marker for primary local activation of innate immunity and inflammatory response [28,29,30] and is highly expressed in atherosclerotic lesions and in plasma from patients with arterial inflammation. In addition, this marker has shown a deleterious effect on isolated human endothelial cells [31] and a negative correlation with surrogate biomarkers of endothelial dysfunction, such as circulating asymmetric dimethylarginine (ADMA) and arginine, in patients with end-stage chronic renal disease [32]. Although the role of PTX3 is not understood completely, these findings may suggest a key role of PTX3 in cardiovascular diseases through a direct effect on endothelial homeostasis [33]. The increase in inflammatory molecules released in the first trimester of pregnancy causes the activation of a cascade of systemic factors, leading to an imbalance between vasodilator and vasoconstrictor molecules. Ultimately, this imbalance gives rise to the clinical manifestation of pre-eclampsia in the second or third trimester. This endothelial dysfunction leads to the activation and secretion of inflammatory factors, such as cytokines and growth factors, in the systemic maternal circulation [34, 35]. Our results are in line with the hypothesis that pre-eclampsia represents the clinical manifestation of endothelial dysfunction as part of an excessive maternal inflammatory response to pregnancy [36]. In addition, PTX3 may represent an inflammatory molecule involved in this complex mechanism, especially for severe stages of the disease. We show in a robust sample size that increased plasma of PTX3 has a stronger association with HELLP syndrome. Cakmak et al. described higher levels of this marker in women with severe pre-eclampsia versus those with mild disease; however, the pre-eclamptic group was small (~100 patients), and no association measurement was calculated for disease severity [37]. More recent studies have revealed that circulating levels of PTX3 were already increased in the first trimester in women who subsequently developed pre-eclampsia, suggesting that PTX3 may be an early predictive factor for PE. These findings also support an etiologic hypothesis for pre-eclampsia being an excessive maternal inflammatory response to pregnancy [38, 39]. However, the fact that we did not observe a dose-dependent response suggests no etiologic role of PTX3 in pre-eclampsia. Clearly, observational studies are not designed to assess relationships beyond associations, but observing dose-dependent responses could hint at causality [40]. Another possibility is that measurement of PTX3 levels could be useful for classifying the severity of the disease rather than as a predictive factor, or even to study this disease in patients with advanced age, a context in which animal models have suggested unconventional pathogenesis of hypertension diseases in pregnancy [41]. Our study has several strengths, especially the sample size (three-fold more evidence than that provided by all previous studies), the population-based design and the extensive information on a large number of potential confounders. In addition, all cases and controls were validated by an outcome committee composed of epidemiologists and consultant obstetricians to further minimize outcome misclassification. The robust sample size allowed us to do subgroup analysis according to time of onset, and although an association with risk was observed, the effect did not vary between early, intermediate and late pre-eclampsia, in contrast to what was reported by Hamad et al. [42]. However, the study also has some limitations: although we adjusted our analyses by many confounders, we cannot rule out the possibility of residual confounding and perhaps reverse causation. In a sample of pregnant Colombian women, we found that PTX3 level was associated with pre-eclampsia and that this association was stronger for HELLP syndrome. Further longitudinal studies and Mendelian randomization analyses would bring more insight into the role of PTX3 in pre-eclampsia.
References
Ronsmans C, Graham WJ. Maternal mortality: who, when, where, and why. Lancet. 2006;368:1189–200.
Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74.
Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol. 2013;170:1–7.
Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99.
Levine R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Ph D. Circulating angiogenic factors and the risk of preeclampsia. N. Engl J Med. 2004;350:672–83.
Borzychowski AM, Sargent IL, Redman CWG. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–16.
Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–66.
Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–93.
Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, et al. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–93.
Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–7.
Fazzini F, Peri G, Doni A, Dell’Antonio G, Dal Cin E, Bozzolo E, et al. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001;44:2841–50.
Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2009;29:594–9.
Zhou P, Luo X, Qi H-B, Zong W-J, Zhang H, Liu D-D, et al. The expression of pentraxin 3 and tumor necrosis factor-alpha is increased in preeclamptic placental tissue and maternal serum. Inflamm Res. 2012;61:1005–12.
Tranguch S, Chakrabarty A, Guo Y, Wang H, Dey SK. Maternal pentraxin 3 deficiency compromises implantation in mice. Biol Reprod. 2007;77:425–32.
Cetin I, Cozzi V, Pasqualini F, Nebuloni M, Garlanda C, Scd V, et al. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006;3:1347–53.
Rovere-Querini P, Antonacci S, Antonio GD, Angeli A. Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet Gynecol. 2006;108:148–55.
Garg P, Kumar A, Kachhawa G, Kumar K. Estimation of asymmetric dimethylarginine (ADMA), placental growth factor (PLGF) and pentraxin 3 (PTX 3) in women with preeclampsia. Pregnancy Hypertens. 2018;14:245–51.
Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981–91.
Barton JR, Sibai BM. Diagnosis and management of hemolysis, elevated liver enzymes, and low platelets syndrome. Clin Perinatol. 2004;31:807–33.
Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2:72–83.
Cozzi V, Garlanda C, Nebuloni M, Maina V, Martinelli A, Calabrese S, et al. PTX3 as a potential endothelial dysfunction biomarker for severity of preeclampsia and IUGR. Placenta. 2012;33:1039–44.
Estudio de Genes Candidatos en Preeclampsia. 2005. Disponible en: www.genpe.org
National High Blood Pressure Education Program. “Report of the national high blood pressure education program working group on high blood pressure in pregnancy”. Am J Obstet Gynecol. 2000;183:s1–22.
Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162:311–6.
Departamento Administrativo Nacional de Estadística. Estratificación Socioeconómica. 2019. Disponible en: https://www.dane.gov.co/files/geoestadistica/Preguntas_frecuentes_estratificacion.pdf.
GenPE. Recruitment questionaire. 2010. Disponible en: http://www.genpe.org//index.php?option=com_content&task=view&id=41&Itemid=26.
Papaioannou TG, Protogerou AD, Vrachatis D, Konstantonis G, Aissopou E, Argyris A, et al. Mean arterial pressure values calculated using seven different methods and their associations with target organ deterioration in a single-center study of 1878 individuals. Hypertens Res. 2016;39:640–7.
Mantovani A, Garlanda C, Bottazzi B, Peri G, Doni A, Martinez Y. et al. The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol. 2006;45:326–30.
Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Pizzetti F, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–41.
Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–54.
Carrizzo A, Procaccini C, Lenzi P, Fusco C, Villa F, Migliarino S, et al. PTX3: An inflammatory protein modulating ultrastructure and bioenergetics of human endothelial cells. Immun Ageing. 2019;16:3–7.
Witasp A, Rydén M, Carrero JJ, Qureshi AR, Nordfors L, Näslund E, et al. Elevated circulating levels and tissue expression of Pentraxin 3 in uremia: a reflection of endothelial dysfunction. PLoS ONE. 2013;8:e63493.
Fornai F, Carrizzo A, Forte M, Ambrosio M, Damato A, Ferrucci M, et al. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immun Ageing. 2016;13:1–9.
Benyo DF, Smarason A, Redman CWG, Sims C, Conrad KP, Obstetrics D, et al. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2014;86:2505–12.
Rinehart BK, Terrone DA, Lagoo-deenadayalan S, William H, Hale EA, Martin JN, et al. Expression of the placental cytokines tumor necrosis factor α, interleukin 1 β, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–20.
Pardi G. Diagnostic value of blood sampling in fetuses with growth retardation. Fetal Diagn Ther. 1993;328:601–2.
Cakmak HA, Cakmak BD, Yayla CA, Coskun I, Erturk M, Keles I. Hypertension in Pregnancy Assessment of relationships between novel inflammatory markers and presence and severity of preeclampsia: epicardial fat thickness, pentraxin-3, and neutrophil-to-lymphocyte ratio. Hypertens Pregnancy. 2017;36:233–9.
Akolekar R, Casagrandi D, Livanos P, Tetteh A, Nicolaides KH. Maternal plasma pentraxin 3 at 11 to 13 weeks of gestation in hypertensive disorders of pregnancy. Prenat Diagn. 2009;29:934–8.
Cetin I, Cozzi V, Papageorghiou AT, Maina V, Montanelli A, Garlanda C, et al. First trimester PTX3 levels in women who subsequently develop preeclampsia and fetal growth restriction. Acta Obstet Gynecol Scand. 2009;88:846–9.
Höfler M. The Bradford Hill considerations on causality: a counterfactual perspective? Emerg Themes Epidemiol. 2005;2:11.
Furuya K, Kumasawa K, Nakamura H, Nishimori K, Kimura T. Novel biomarker profiles in experimental aged maternal mice with hypertensive disorders of pregnancy. Hypertens Res. 2019;42:29–39.
Hamad RR, Eriksson MJ, Berg E, Larsson A, Bremme K. Impaired endothelial function and elevated levels of pentraxin 3 in early-onset preeclampsia. Acta Obstet Gynecol Scand. 2012;91:50–6.
Funding
This work was supported by project grants from Departamento Administrativo de Ciencia, Tecnología e Innovación, Colciencias - Colombia [Grant number 14134319235]; and Universidad Autónoma de Bucaramanga - UNAB [Grant number EGEN22].
Author information
Authors and Affiliations
Contributions
NSD and JPC are principal investigators who designed the GenPE study and led the idea for this paper. CCCM performed data analysis, interpretation and writing of the draft manuscript. DCQL and PKBN supported the data analysis and interpretation of the results. PBN and EGM performed sample analysis for pentraxin-3 measurements. MCPL was a national recruitment coordinator and made quality control of clinical information with LADM and MLL. MBN, ROS, AMC, CMR, GM, ESB, and WS were responsible for patient recruitment and data acquisition. All authors have made substantial contributions to the content of this paper and have read and approved the submission of the manuscript. The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Colmenares-Mejía, C.C., Quintero-Lesmes, D.C., Bautista-Niño, P.K. et al. Pentraxin-3 is a candidate biomarker on the spectrum of severity from pre-eclampsia to HELLP syndrome: GenPE study. Hypertens Res 43, 884–891 (2020). https://doi.org/10.1038/s41440-020-0434-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0434-0
This article is cited by
-
Preeclampsia up to date—What’s going on?
Hypertension Research (2023)
-
Diagnostic biomolecules and combination therapy for pre-eclampsia
Reproductive Biology and Endocrinology (2022)
-
Melatonin and gestational hypertension
Hypertension Research (2021)
-
Pentraxin-3 and the pathogenesis of preeclampsia
Hypertension Research (2020)