Abstract
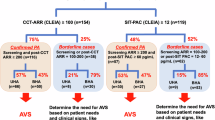
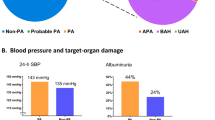
The new Japanese guidelines for primary aldosteronism introduce a category in the judgment of functional confirmatory tests that is called the “borderline range,” which is rare in the other international guidelines. The clinical characteristics of this borderline group are not yet understood. To investigate whether this borderline group has any significant differences in terms of target organ damage, we used data from a Japanese nationwide registry (JPAS-II) of individuals with primary aldosteronism or essential hypertension to compare the borderline group with the definitive-positive group and the negative group. We analyzed the cases of 1785 patients based on their captopril-challenge test results. Since the JPAS-II database contains plasma aldosterone concentration values obtained based on both radioimmunoassay (n = 1555) and chemiluminescent enzyme immunoassay (n = 230) principles, we converted these values to their equivalents as if measured by chemiluminescent enzyme immunoassay and conducted all analyses under the simulated condition. Multicovariate-adjusted models revealed significant prevalance odds ratios for chronic kidney disease (2.01, 95% confidence interval: 1.13 to 3.61), electrocardiographic abnormalities (1.66, 95% confidence interval: 1.16 to 2.37). No significant difference was observed between the borderline and negative groups in these assessments (odds ratio [95% confidence interval] for chronic kidney disease: 0.73 [0.26 to 2.02] and electrocardiographic abnormalities: 1.01 [0.60 to 1.70]). We confirmed that the prevalence of target organ damage increases linearly as the aldosterone-to-renin ratio rises following the captopril challenge test. These results provide material to consider regarding the significance of the provisionally established borderline group.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Conn JW, Knopf RF, Nesbit RM. Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg. 1964;107:159–72.
Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–20.
Naruse M, Katabami T, Shibata H, Sone M, Takahashi K, Tanabe A, et al. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr J. 2022;69:327–59.
Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9:459–69.
Bianchi S, Batini V, Bigazzi R. The renal effects of mineralocorticoid receptor antagonists. Int J Cardiol. 2015;200:20–24.
Manosroi W, Atthakomol P, Wattanawitawas P, Buranapin S. Differences in glycemic abnormalities between primary aldosteronism and essential hypertension: a systematic review and meta-analysis. Front Endocrinol. 2022;13:870047.
Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep. 2011;13:163–72.
Haze T, Hatakeyama M, Komiya S, Kawano R, Ohki Y, Suzuki S, et al. Association of the ratio of visceral-to-subcutaneous fat volume with renal function among patients with primary aldosteronism. Hypertens Res. 2021;44:1341–51.
Haze T, Ozawa M, Kawano R, Haruna A, Ohki Y, Suzuki S, et al. Effect of the interaction between the visceral-to-subcutaneous fat ratio and aldosterone on cardiac function in patients with primary aldosteronism. Hypertens Res. 2023;46:1132–44.
Chen SY, Chen JY, Huang WC, Puar THK, Chin Kek P, Chueh JS, et al. Cardiovascular outcomes and all-cause mortality in primary aldosteronism after adrenalectomy or mineralocorticoid receptor antagonist treatment: a meta-analysis. Eur J Endocrinol. 2022;187:S47–S58.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–66.
Haze T, Tamura K. Possible relationship between primary aldosteronism and small vessel disease. Hypertens Res. 2023;47:677–78.
Qian N, Xu J, Wang Y. Stroke risks in primary aldosteronism with different treatments: a systematic review and meta-analysis. J Cardiovasc Dev Dis. 2022;9:300.
Wu V-C, Wang S-M, Huang K-H, Tsai YC, Chan C-K, Yang S-Y, et al. Long-term mortality and cardiovascular events in patients with unilateral primary aldosteronism after targeted treatments. Eur J Endocrinol. 2021;186:195–205.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Amar L, Baguet JP, Bardet S, Chaffanjon P, Chamontin B, Douillard C, et al. SFE/SFHTA/AFCE primary aldosteronism consensus: introduction and handbook. Ann Endocrinol. 2016;77:179–86.
Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, et al. Guidelines for the diagnosis and treatment of primary aldosteronism–the Japan Endocrine Society 2009. Endocr J. 2011;58:711–21.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Kobayashi H, Nakamura Y, Abe M, Tanabe A, Sone M, Katabami T, et al. Impact of a change to a novel chemiluminescent immunoassay for measuring plasma aldosterone on the diagnosis of primary aldosteronism. Endocr J. 2023;70:489–500.
Kobayashi H, Nakamura Y, Abe M, Nakamura T, Nozato Y, Izawa S, et al. Prevalence of unilateral hyperaldosteronism in primary aldosteronism: impact of a novel chemiluminescent immunoassay for measuring plasma aldosterone in Japan. Hypertens Res. 2024; https://doi.org/10.1038/s41440-024-01786-5.
Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension. 2018;71:530–37.
Haze T, Yano Y, Hatano Y, Tamura K, Kurihara I, Kobayashi H, et al. Association of achieved blood pressure after treatment for primary aldosteronism with long-term kidney function. J Hum Hypertens. 2022;36:904–10.
Kobayashi Y, Haze T, Yano Y, Tamura K, Kurihara I, Ichijo T, et al. Associations between changes in plasma renin activity and aldosterone concentrations and changes in kidney function after treatment for primary aldosteronism. Kidney Int Rep. 2020;5:1291–97.
Haze T, Hirawa N, Yano Y, Tamura K, Kurihara I, Kobayashi H, et al. Association of aldosterone and blood pressure with the risk for cardiovascular events after treatments in primary aldosteronism. Atherosclerosis. 2021;324:84–90.
Mulatero P, Paglieri C, Morello F, Chiandussi L. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002; 40:897–902.
Hua KF, Wu YH, Zhang ST. Clinical diagnostic value of liquid chromatography-tandem mass spectrometry method for primary aldosteronism in patients with hypertension: a systematic review and meta-analysis. Front Endocrinol. 2022;13:1032070.
Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
White SL, Yu R, Craig JC, Polkinghorne KR, Atkins RC, Chadban SJ. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis. 2011;58:19–28.
Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, et al. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int. 2006;69:1264–71.
Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–99.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–99.
Guo Z, Poglitsch M, McWhinney BC, Ungerer JPJ, Ahmed AH, Gordon RD, et al. Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. 2018;103:3965–73.
Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39:1057–88.
Lim PO, Struthers AD, MacDonald TM. The neurohormonal natural history of essential hypertension: towards primary or tertiary aldosteronism? J Hypertens. 2002;20:11–15.
Neves MF, Schiffrin EL. Aldosterone: a risk factor for vascular disease. Curr Hypertens Rep. 2003;5:59–65.
Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, März W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31:1237–47.
Nanba K, Tsuiki M, Nakao K, Nanba A, Usui T, Tagami T, et al. A subtype prediction score for primary aldosteronism. J Hum Hypertens. 2014;28:716–20.
Wada N, Miyoshi A, Usubuchi H, Terae S, Shibayama Y, Takahashi B, et al. Prediction of unilateral hyperaldosteronism on adrenal vein sampling using captopril challenge test in patients with primary aldosteronism. Endocr J. 2021;68:45–51.
Kobayashi H, Haketa A, Ueno T, Ikeda Y, Hatanaka Y, Tanaka S, et al. Scoring system for the diagnosis of bilateral primary aldosteronism in the outpatient setting before adrenal venous sampling. Clin Endocrinol. 2017;86:467–72.
Weigel M, Riester A, Hanslik G, Lang K, Willenberg HS, Endres S, et al. Post-saline infusion test aldosterone levels indicate severity and outcome in primary aldosteronism. Eur J Endocrinol. 2015;172:443–50.
Wu C-H, Wu V, Yang Y-W, Lin Y-H, Yang S-Y, Lin P-C, et al. Plasma aldosterone after seated saline infusion test outperforms captopril test at predicting clinical outcomes after adrenalectomy for primary aldosteronism. Am J Hypertens. 2019;32:1066–74.
Xiang Q, Wang W, Chen T, Yu K, Li Q, Zhang T, et al. The value of the post-captopril aldosterone/renin ratio for the diagnosis of primary aldosteronism and the influential factors: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2020;21:1470320320972032.
KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2024;105:s117–s314.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e95.
Ha J, Park JH, Kim KJ, Kim JH, Jung KY, Lee J, et al. 2023 Korean Endocrine Society consensus guidelines for the diagnosis and management of primary aldosteronism. Endocrinol Metab. 2023;38:597–618.
Acknowledgements
We express our gratitude to all JPAS-II investigators.
JPAS II study group
Masakatsu Sone15, Kenichi Yokota15, Takuyuki Katabami17, Mitsuhide Naruse16, Keiichiro Nakamae16, Toshifumi Nakamura11, Akiyo Tanabe18, Daisuke Taura6, Yoshihiro Ogawa19, Kouichi Yamamoto20, Takashi Yoneda21, Mitsuhiro Kometani21, Tetsuya Yamada14, Masanori Murakami14, Katsutoshi Takahashi22, Hiroki Kobayashi10, Takamasa Ichijo7, Norio Wada13, Kohei Kamemura5, Yuichi Fujii23, Yuichiro Yoshikawa24, Shintaro Okamura25, Shigeatsu Hashimoto26, Minemori Watanabe9, Shoichiro Izawa12, Mika Tsuiki4, Hiromasa Goto27, Miki Kakutani28, Kouichi Tamura1, Nobuhito Hirawa3, Takehiro Kato29, Yutaka Takahashi8, Ryuji Okamoto30, Kazutoshi Miyashita31, Kihei Yoneyama32, Michio Otsuki33
Funding
JPAS and JRAS were supported by the research grant from the Japan Agency for Medical Research and Development (AMED) under Grant number JP17ek0109122 and JP20ek0109352. JPAS-II was partly supported by a Grant-in-Aid from the Ministry of Health, Labour, and Welfare, Japan (No. 23FC0201 for research on intractable adrenal disorders).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujiwara, N., Haze, T., Wakui, H. et al. Differences in target organ damage between captopril challenge test-defined definitive-positive and borderline-range groups among patients with primary aldosteronism. Hypertens Res 48, 540–552 (2025). https://doi.org/10.1038/s41440-024-01943-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01943-w