Abstract
This study aimed to evaluate the association between hypertension (HT) onset age and later risks of chronic kidney diseases (CKD) and mortality. Adult patients without CKD from 2008 to 2013 were identified using electronic medical records from United Kingdom (UK) and Hong Kong (HK). Patients newly diagnosed with HT and those without were included in the HT and control groups, respectively. All subjects were stratified into six age groups (18-39, 40-49, 50-59, 60-69, 70-79, ≥80). Multivariable Cox proportional hazard regression, adjusted with baseline characteristics and fine stratification weights, was conducted to investigate the association between HT onset and risks of CKD, renal decline, end-stage renal disease (ESRD), and all-cause mortality. Subjects were followed up from baseline until an outcome event, death, or administrative end of the cohort, whichever occurred first. A total of 4,413,551 and 3,132,951 subjects were included in the UK and HK cohorts, respectively. HT was significantly associated with increased risks of outcome, but the hazard ratios (HRs) decreased with increasing onset age. In the UK cohort, the HRs (95% confidence intervals) for subjects aged 18-39 and ≥80 were 3.69 (3.53, 3.86) and 2.01 (1.96, 2.06) for CKD, 3.83 (3.60, 4.07) and 3.17 (2.97, 3.38) for renal decline, 17.26 (14.34, 20.77) and 2.55 (2.12, 3.07) for ESRD, 2.88 (2.66, 3.11) and 1.09 (1.07, 1.12) for mortality. The HK cohort exhibited a similar pattern. Our study concluded that early onset of HT significantly affects renal health later in life, while the contribution decreases with the onset age of HT.

Similar content being viewed by others
Introduction
In 2010, there was an estimated 1.39 billion people with hypertension (HT) (i.e. defined as having systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg) representing over 30% of the global population [1], and this number is only rapidly rising [2]. As the leading risk factor for chronic comorbidities and mortality, high blood pressure was associated with 7.8 million all-cause deaths, accounting for 14.0% of all deaths, in 2015 [3]. HT has also been shown to be correlated with the risks of chronic kidney disease (CKD) and end-stage renal disease (ESRD), and this correlation is dose-responsive and continuous for systolic blood pressure >120 mmHg [4,5,6,7]. Globally, the age of HT onset has been progressively decreasing, leading to increasing incidence of HT among young adults likely because of changing psychosocial and lifestyle factors [8]. These trends have placed growing burdens on scarce resources and strained health systems [9]. To date, there is limited evidence examining the effects of onset age for HT on the risks of CKD and mortality. Most of the existing evidence has centered on cardiovascular morbidity and mortality, in which early-onset HT has been found to increase the risk of cardiovascular and target end-organ damage as well as mortality [10,11,12]. Renal complications, as a specific secondary disease outcome, have rarely been studied, despite its heavy disease burden.
Therefore, we investigated the associations of new-onset HT among different age groups with CKD risks and mortality in two large community-based cohorts from Hong Kong and the UK. The value of this study lies in its potential to implications informing on health policies and guidelines to support the prevention and management of HT, given its heritable nature and the priorities of health systems on the optimal management of HT including treatment of its sequelae [11, 13, 14].
Methods
Study design
This retrospective cohort study was designed using electronic health records from the United Kingdom (UK) and Hong Kong (HK), which were collected during routine clinical practice within their healthcare systems. The two cohorts included patients aged 18 and above who attended health services from 2008 to 2013. For the UK cohort, records were extracted from The Health Improvement Network (THIN), one of the most respected and reliable primary care data sources, representing around 6% of the UK population since 1994 [15, 16]. The electronic health records in Hong Kong were provided by the Hospital Authority (HA), a statutory body managing the majority of public outpatient services and all public inpatient services, encompassing 43 public hospitals, 49 specialist outpatient clinics, and 74 general outpatient clinics [17,18,19,20]. In Hong Kong, all residents have access to the public healthcare services provided by the government. The HA database provides real-time updated clinical information for routine clinical practice, including patients’ demographic information, diagnoses, medication prescriptions, and laboratory test results. Additionally, death records in Hong Kong were obtained from the Deaths Registry, a government-operated institution that maintains data on all registered deaths in Hong Kong. These death records were then linked with the HA database using anonymised unique patient identifiers. The ethnic composition of the two cohorts differs: 87% of the UK population is Caucasian [21], while in HK, 90% of the population is Chinese [22]. The data quality and the coding accuracy of the two databases have been demonstrated in many previous, high-quality epidemiological studies [23, 24].
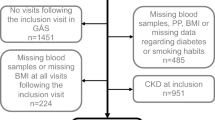
We included patients aged 18 and above who attended public health services from January 2008 to December 2013, using electronic healthcare records from the UK and HK. Subjects newly diagnosed with HT or prescribed antihypertensive drugs were classified as the onset HT group. In contrast, patients who attended public health services during the study period without a history of HT diagnosis or antihypertensive drug use were identified as controls. The index date was defined as the date of HT onset for the onset HT group, and the first date of public health services attendance during the inclusion period for the control group. A flowchart of the subject selection process is presented in Fig. 1. In the UK cohort, HT was defined using the Read Code (Supplementary Table 1), and in the HK cohort, it was defined using the International Classification of Primary Care, Second Edition (ICPC-2) codes (K86, K87) and the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes (401*, 402*, 403*, 404*, 405*). Prescriptions of antihypertensive drugs were extracted using British National Formulary (BNF) codes 2.5.5*, 2.4*, 2.6.2*, and 2.2* in both cohorts. Patients diagnosed with CKD at the start of the study were excluded. To detect most of the CKD incidence during follow-up, CKD onset was defined by diagnosis records (Read Code in Supplementary Table 1 for the UK database, ICD-9-CM codes 585.3-585.6 or 586 for the HK database) or by an eGFR test of less than 60 mL/min/1.73 m², estimated by the abbreviated Modification of Diet in Renal Disease Study formula. The Read Codes for CKD were referenced from LSHTM Data Compass [25]. The ICD-9-CM and ICPC-2 codes for defining CKD used were referenced from previous studies using the same database [26, 27]. The follow-up period was from the index date until the outcome event, death, transfer out of the healthcare practice, or the administrative end of the cohort (HK cohort: December 31, 2018; UK cohort: December 31, 2019), whichever occurred first.
Outcome measures
Outcomes related to renal disease included new onset of ESRD, CKD, and renal decline after baseline. ESRD was defined by the Read Code, ICD-9-CM codes (585.5, 585.6, 586), or an eGFR of <15 mL/min/1.73 m². Renal decline was defined as a drop of at least 30% in eGFR from baseline. Other outcomes of interest included all-cause mortality. Read Codes for identifying renal disease outcomes are listed in Supplementary Table 1. Death records were identified by a valid death date in both cohorts.
Baseline characteristics
Baseline characteristics included age, gender, smoking status, Charlson comorbidity index, prescription of lipid-lowering drugs and antidiabetic drugs, and the presence of obesity, diabetes mellitus, atrial fibrillation, peripheral vascular disease, amputation, dementia, chronic lung disease, connective tissue disease, peptic ulcer, liver disease, cardiovascular disease, hemiplegia, leukaemia, malignant lymphoma, and cancer. The Charlson Comorbidity Index was calculated based on age and a range of concurrent conditions (i.e., myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, hemiplegia or paraplegia, diabetes, renal disease, liver disease, peptic ulcer disease, rheumatologic disease, malignancy, and AIDS) to estimate the long-term mortality risk [28].
Statistical analysis
After inclusion and exclusion, subjects were divided into six age groups (18-39, 40-49, 50-59, 60-69, 70-79, ≥80 years). The risk of outcomes was compared between the onset HT group and the control group within each age stratum. To ensure balance of baseline characteristics between the compared groups, fine stratification weights were estimated for each subject based on propensity scores. Propensity scores were estimated based on multivariate logistic regression, considering subjects’ characteristics at baseline, including age, gender, smoking status, Charlson comorbidity index, obesity, diabetes mellitus, atrial fibrillation, peripheral vascular disease, amputation, dementia, chronic lung disease, connective tissue disease, peptic ulcer, liver disease, cardiovascular disease, hemiplegia, leukaemia, malignant lymphoma, cancer, and the use of lipid-lowering and antidiabetic drugs. Subjects were stratified and weighted within each of the fifty quantile categories of the propensity score. After weighting, the standardized mean difference (SMD) was calculated to assess the balance of baseline covariates between the exposure and control groups. An SMD of less than 0.2 indicates sufficient balance [29].
Data were presented as mean and standard deviation for continuous variables, or count and percentage for categorical variables. Within each age stratum, incidence rates and their corresponding confidence intervals (CIs) were estimated for the onset HT and control groups separately, based on the Poisson distribution. Cox proportional hazards regression, adjusted for patients’ baseline characteristics and weighting, was further conducted to investigate the association between new-onset HT and the risk of each outcome.
Subgroup analysis was performed on gender to explore potential heterogeneity of the associations between male and female as identified in the literature [30]. Subjects were stratified by gender within each age group, and fine stratification weights were estimated separately in each subgroup. Multiple sensitivity analyses were conducted to test the robustness of the results. First, subjects who experienced an outcome or died during the first year of follow-up were excluded. Second, exact one-to-one matching based on age and sex was applied instead of fine stratification weighting. Third, subjects in the control group diagnosed with HT during the first year of follow-up were excluded from the analysis. Fourth, controls diagnosed with HT during the entire follow-up period were excluded. Fifth, one-to-one matching based on propensity score, rather than fine stratification weights, was used. Sixth, controls with HT diagnosed after baseline were censored. Seventh, subjects’ body mass index (BMI) and estimated glomerular filtration rate (eGFR) at baseline were adjusted as covariates in the logistic model for estimating fine stratification weights and the Cox regression. Multiple imputation was applied to handle missing values for these parameters, ensuring data completeness and statistical power. Eighth, in accordance with the latest recommendations from international guidelines, two eGFR measurements taken at least 3 months apart were used to identify cases of CKD and ESRD, instead of relying on a single eGFR measurement [31, 32]. Specifically, CKD was redefined as either 1) a diagnosis of CKD confirmed by a doctor or 2) two eGFR measurements of less than 60 mL/min/1.73 m². Similarly, ESRD was redefined as either a diagnosis of ESRD or two eGFR measurements of less than 15 mL/min/1.73 m². Ninth, subjects in the onset HT group with available baseline blood pressure measurements were stratified into three hypertension grades according to statements from the World Health Organization [33] and guidelines from European Society of Cardiology/European Society of Hypertension [34] (grade 1: systolic blood pressure (SBP) 140-159 mmHg and/or diastolic blood pressure (DBP) 90-99 mmHg; grade 2: SBP 160-179 mmHg and/or DBP 100-109 mmHg; grade 3: SBP ≥ 180 mmHg and/or DBP ≥ 110 mmHg). They were separately compared to subjects withourt HT in the same age group.
All statistical analyses were conducted using Stata 15.1, applying two-tailed significance tests with a significance level of p < 0.05.
Results
A total of 514,161 and 579,138 subjects with HT, and 3,899,390 and 2,553,813 subjects without HT, were identified in the UK and HK cohorts, respectively (Fig. 1). Details regarding the distribution of subjects’ baseline characteristics before and after weighting are provided in Supplementary Tables 2–5. The SMD values after weighting were <0.2 for all variables, indicating sufficient balance. After a median follow-up of 11.3 years (around 44 million person-years) for the UK cohort and 9.2 years (around 31 million person-years) for the HK cohort, a total of 207,474 and 151,240 CKD, 42,829 and 240,051 renal decline, 3,164 and 16,249 ESRD, and 114,134 and 162,741 all-cause mortality events were recorded, respectively.
The incidence rates (IRs) of each outcome are listed in Table 1. In the UK cohort, the IR (cases/1000 person-years) with 95% confidence interval for CKD, renal decline, ESRD and all-cause mortality were higher in the new-onset HT group than in the control group for subjects aged 18-39 at the index date (CKD, IR: 2.87 [2.76, 2.97] vs. 0.62 [0.61, 0.63]; renal decline: 1.53 [1.46, 1.61] vs. 0.28 [0.27, 0.29]; ESRD: 0.21 [0.18, 0.24] vs. 0.01 [0.01, 0.01]; all-cause mortality: 0.86 [0.80, 0.92] vs. 0.22 [0.22, 0.23]). For those aged 80 and above at baseline, the incidence of all outcomes increased but the IRs remained higher in the new-onset HT group compared to the controls (CKD, IR: 100.77 [99.27, 102.29] vs. 36.93 [36.28, 37.60]; renal decline: 12.62 [12.19, 13.07] vs. 2.93 [2.76, 3.10]; ESRD: 1.27 [1.14, 1.41] vs. 0.48 [0.41, 0.55]; all-cause mortality: 65.06 [64.08, 66.05] vs. 50.44 [49.74, 51.15]). Results on the hazard ratios (HRs) for all outcomes are shown in Table 1 and Fig. 2. Subjects with HT had significantly increased risks of CKD, renal decline, ESRD, and mortality across all age groups compared to those without HT; however, the magnitude of the relative risks decreased with increasing age of onset. For example, the HR (95% CI) for renal decline was 3.83 (3.60, 4.07) for subjects with new-onset HT compared to those without in subjects aged 18-39 years, while this HR (95% CI) estimated for the group with baseline age ≥80 years decreased to 3.17 (2.97, 3.38). Similarly, the estimated hazard ratios (HRs [95% CIs]) for CKD and ESRD were higher in subjects aged 18-39 years (CKD: 3.69 [3.53, 3.86]; ESRD: HR 17.26 [14.34, 20.77]) than in subjects aged ≥80 years (CKD: 2.01 [1.96, 2.06]; ESRD: HR 2.55 [2.12, 3.07]). The results for the HK cohort showed similar results. The IRs (cases/1000 person-years) with 95% confidence interval for subjects aged 18-39 years were 4.82 (4.61, 5.04) and 0.50 (0.49, 0.51) for CKD, 25.29 (24.78, 25.81) and 2.41 (2.38, 2.44) for renal decline, 1.01 (0.92, 1.11) and 0.07 (0.07, 0.08) for ESRD, and 3.62 (3.44, 3.81) and 0.60 (0.59, 0.62) for all-cause mortality for the subjects with new onset of HT and controls, respectively. For subjects aged ≥ 80 years, the IRs (cases/1000 person-years) with 95% confidence interval were 101.76 (100.40, 103.14) and 52.92 (51.93, 53.92) for CKD, 110.91 (109.49, 112.36) and 40.62 (39.77, 41.49) for renal decline, 7.34 (7.01, 7.68) and 4.10 (3.85, 4.38) for ESRD, and 102.56 (101.32, 103.80) and 71.22 (70.13, 72.33) for all-cause mortality for the subjects with new onset of HT and controls, respectively. The HR (95% CI) for renal decline was 8.74 (8.53, 8.97) for subjects with HT compared to those without at age 18-39 years, while the HR decreased to 2.14 (2.08, 2.19) for subjects with HT compared to those without at age ≥80 years.
The association between onset of hypertension and kidney disease/mortality in different age groups using Cox regression. Hazard ratio with 95% confidence interval adjusted by age, gender, smoking status, Charlson comorbidity index, obesity, diabetes mellitus, atrial fibrillation, peripheral vascular disease, amputation, dementia, chronic lung disease, connective tissue disease, peptic ulcer, liver disease, cardiovascular disease, hemiplegia, leukaemia, malignant lymphoma, cancer, the use of anti-diabetic drugs, lipid-lowering agents, and weighting. CKD Chronic kidney disease, ESRD End-stage renal disease CI Confidence interval
Association between new onset of hypertension and risk of kidney disease/mortality within subgroups stratified by gender. Hazard ratio with 95% confidence interval adjusted by age, smoking status, Charlson comorbidity index, obesity, diabetes mellitus, atrial fibrillation, peripheral vascular disease, amputation, dementia, chronic lung disease, connective tissue disease, peptic ulcer, liver disease, cardiovascular disease, hemiplegia, leukaemia, malignant lymphoma, cancer, the use of anti-diabetic drugs, lipid-lowering agents, and weighting. CKD Chronic kidney disease, HT Hypertension, ESRD End-stage renal disease, HR Hazard Ratio, CI Confidence interval
Results for subgroup analysis are presented in Fig. 3. The HRs (95% CIs) for CKD, renal decline, and all-cause mortality were significantly higher in males compared to females among individuals aged 18-39 in both the UK cohort (CKD: 4.91 [4.60, 5.24] for male, 3.00 [2.83, 3.19] for female, p < 0.05 for heterogeneity test; renal decline: 4.33 [3.93, 4.76] for male, 3.52 [3.25, 3.81] for female, p < 0.05 for heterogeneity test; all-cause mortality: 3.08 [2.80, 3.39] for male, 2.48 [2.15, 2.86] for female, p < 0.05 for heterogeneity test) and HK cohort (CKD: 6.23 [5.74, 6.77] for male, 5.23 [4.72, 5.79] for female, p < 0.05 for heterogeneity test; renal decline: 10.7 [10.2, 11.1] for male, 7.86 [7.61, 8.11] for female, p < 0.05 for heterogeneity test; all-cause mortality: 3.89 [3.56, 4.25] for male, 2.73 [2.40, 3.09] for female, p < 0.05 for heterogeneity test). While this difference was not apparent in the ≥80 age group, the HRs (95% CIs) for renal decline were relatively higher in female than male in both cohorts (UK cohort: 2.79 [2.54, 3.06] for male, 3.48 [3.18, 3.81] for female, p < 0.05 for heterogeneity test; HK cohort: 2.04 [1.96, 2.12] for male, 2.21 [2.14, 2.28] for female, p < 0.05 for heterogeneity test). The results of all sensitivity analyses are presented in Supplementary Figs. 1–8 and Supplementary Table 7, all of which demonstrated a similar pattern (i.e. the HRs decreased with increasing onset age) to the main findings. Notably, as shown in Supplementary Fig. 1, the HR (95% CI) for ESRD in subjects with at least 1-year of follow-up and aged 18-39 years was 6.28 (4.67, 8.45) in the UK cohort, which was relatively lower compared to the HR of 17.26 (14.34, 20.77) observed in the same age group in the main findings (Fig. 2). Slightly higher HRs (95% CIs) for CKD and ESRD were also observed when defining these two outcomes using two eGFR measurements instead of a single measurement (HR [95% CI] in subjects aged 18-39: UK cohort: 21.66 [17.20,27.26] for ESRD, 5.65 [5.27,6.07] for CKD; HK cohort: 11.73 [9.57,14.38] for ESRD, 11.28 [9.51,13.39] for CKD) (Supplementary Fig. 8).
Discussion
The findings from this cohort study with a large sample of Caucasian and Chinese participants indicated that older adults with new HT onset had significantly higher incidence rates but lower hazard ratios of CKD, renal decline, ESRD, and mortality compared to younger adults. The increased absolute risks with age in both subjects with and without HT onset were also observed in another study on the risk for cardiovascular disease and all-cause mortality [12]. It could be explained by the natural decline in kidney [35] and other organ function [36] through aging. Regarding the relative risk across age groups, the HRs for CKD were 1.5 times higher in the UK and 3.5 times higher in HK among those with HT onset at 18-39 years compared to those ≥80 years. These align with the findings from previous studies particularly in regard to the association between younger HT onset and higher risk of target end-organ damage and mortality [10,11,12]. This highlights the urgent need for HT control starting at a young age as well as for optimal management for all, including both old and young, patients with new onset HT.
The increased relative risks of unfavorable outcomes in those with younger HT onset may be attributed to poorer disease control and lengthier exposure to the chronic disease itself. The baseline clinical characteristics of this study population showed that features such as body mass index and HbA1c were more suboptimal in younger age groups. These risk factors may account for the variation in outcome trends among the age groups. In turn, lower priority of health among other obligations such as study, work, or social life in the younger groups could lead to less engagement in healthy lifestyles and thus poorer control of clinical characteristics at baseline. Evidence has also shown a lack of persistence and continuity in controlling BP once initial treatment interventions are delivered, which may have greater effects on younger patients as they have longer disease courses [37]. Also, as symptoms are less likely to appear in the early stages of HT, younger patients may be less aware and motivated to comply with medical interventions [37].
This study had multiple strengths. First, age-stratified counterparts were used to fully evaluate the effects of HT onset age on CKD risks and mortality, which was further controlled for confounders such as sex and comorbidities. Our study included an adequately large sample size of 1,093,299 participants with and 6,453,203 without HT, which increases the external validity. By extracting data on healthcare services utilization from the representative electronic databases representing all public hospitals and clinics in Hong Kong, the magnitude of health problems relating to the young onset of HT was studied, covering a range of outcomes of clinical. However, there are also several limitations. First, as this is an observational study, causal relationships between onset age and health risks could not be established. Second, classification errors may occur using disease codes or one-time measurement values. We have performed sensitivity analysis using two eGFR measurements to define CKD and ESRD which showed a similar result pattern. Third, potential confounders such as lifestyle (diet and exercise habits) and social factors which may bias the estimation, were not considered in the analysis. Forth, some HT-related outcomes might take longer to develop, hence a longer follow-up period would be beneficial to truly evaluate the effect of onset timing. Fifth, the treatment status after the onset of hypertension and the cause of hypertension were not considered in the current cohort study design.
Conclusion
This cohort study identified the increased risks of renal complications such as CKD and renal decline among patients with early onset HT. Given the growing prevalence of early onset HT across the globe, attention should be paid to the prevention, early detection and control of HT among the young population.
Data availability
The electronic medical records used in the current study are provided by the Hospital Authority of Hong Kong and IQVIA. The data can be accessed upon request to the Hospital Authority of Hong Kong and IQVIA.
References
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. The Lancet. 2005;365:217–23.
Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317:165–82.
Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162:258–65.
Barri YM. Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep. 2008;10:39–45.
Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–8.
Reynolds K, Gu D, Muntner P, Kusek JW, Chen J, Wu X, et al. A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J Am Soc Nephrol. 2007;18:1928–35.
De Venecia T, Lu M, Figueredo VM. Hypertension in young adults. Postgrad Med. 2016;128:201–7.
Chow CK, Gupta R. Blood pressure control: a challenge to global health systems. Lancet. 2019;394:613–5.
Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, et al. Heritability and risks associated with early onset hypertension: multigenerational, prospective analysis in the Framingham Heart Study. BMJ. 2017;357:j1949.
Suvila K, McCabe EL, Lehtonen A, Ebinger JE, Lima JAC, Cheng S, et al. Early Onset Hypertension Is Associated With Hypertensive End-Organ Damage Already by MidLife. Hypertension. 2019;74:305–12.
Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of Age of Onset of Hypertension With Cardiovascular Diseases and Mortality. J Am College Cardiol. 2020;75:2921–30.
World Health Organisation. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis. Geneva, Switzerland; World Health Organisation: 2013.
Perkovic V, Huxley R, Wu Y, Prabhakaran D, MacMahon S. The Burden of Blood Pressure-Related Disease. Hypertension. 2007;50:991–7.
Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Safety. 2007;16:393–401.
Kotz D, O’Donnell A, McPherson S, Thomas KH. Using primary care databases for addiction research: An introduction and overview of strengths and weaknesses. Addictive Behav Rep. 2022;15:100407.
Lau IT. A Clinical Practice Guideline to Guide a System Approach to Diabetes Care in Hong Kong. Diabetes Metab J. 2017;41:81–8.
Liu YZ, Chu R, Lee A, Gomersall CD, Zhang L, Gin T, et al. A surveillance method to identify patients with sepsis from electronic health records in Hong Kong: a single centre retrospective study. BMC Infect Dis. 2020;20:652.
Kong X, Yang Y, Gao J, Guan J, Liu Y, Wang R, et al. Overview of the health care system in Hong Kong and its referential significance to mainland China. J Chin Med Assoc. 2015;78:569–73.
Hospital Authority. Hospital Authority statistical report 2022-2023. 2023. https://www3.ha.org.hk/data/HAStatistics/DownloadReport/6?isPreview=False. Accessed 5 September.
Statistics OfN. 2011 Census: Key Statistics and Quick Statistics for Local Authorities in the United Kingdom. 2011. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/keystatisticsandquickstatisticsforlocalauthoritiesintheunitedkingdom/2013-10-11. Accessed Mar 28.
Census and Statistics Department of Hong Kong. Hong Kong 2016 population by-census summary results. Hong Kong Government Press; 2017.
Myland M, Buysse B, Tsong W, Power GS, Nordli D, Chin RFM. Seizure frequency, healthcare resource utilisation and mortality in childhood epilepsy: a retrospective cohort study using the THIN database. Arch Dis Child. 2019;104:1070–6.
Wei Y, Yan VKC, Kang W, Wong ICK, Castle DJ, Gao L, et al. Association of Long-Acting Injectable Antipsychotics and Oral Antipsychotics With Disease Relapse, Health Care Use, and Adverse Events Among People With Schizophrenia. JAMA Netw Open. 2022;5:e2224163.
Mcdonald H, Evans D, Clay S, Edelstein M, Thomas S. Immunisation risk group - chronic kidney disease. London School of Hygiene & Tropical Medicine. 2020. https://doi.org/10.17037/DATA.00001664.
Jin Q, Mei J, Wong YC, Lam CLK, Wan EYF. Associations and attributable burden between risk factors and all-cause and cause-specific mortality at different ages in patients with hypertension. Hypertens Res. 2024;47:2053–63.
Wan EYF, Yu EYT, Chin WY, Fong DYT, Choi EPH, Lam CLK. Association of Blood Pressure and Risk of Cardiovascular and Chronic Kidney Disease in Hong Kong Hypertensive Patients. Hypertension. 2019;74:331–40.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9.
Austin PC. Some methods of propensity‐score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical J. 2009;51:171–84.
Weldegiorgis M, Woodward M. The impact of hypertension on chronic kidney disease and end-stage renal disease is greater in men than women: a systematic review and meta-analysis. BMC Nephrol. 2020;21:506.
KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105:S117–s314.
Chronic kidney disease: assessment and management (NICE Guideline, No. 203.). 2021. https://www.ncbi.nlm.nih.gov/books/NBK574714/.
Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104.
Jiang S, Sun X, Gu H, Chen Y, Xi C, Qiao X, et al. Age-related change in kidney function, its influencing factors, and association with asymptomatic carotid atherosclerosis in healthy individuals-a 5-year follow-up study. Maturitas. 2012;73:230–8.
Tian YE, Cropley V, Maier AB, Lautenschlager NT, Breakspear M, Zalesky A. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat Med. 2023;29:1221–31.
Hinton TC, Adams ZH, Baker RP, Hope KA, Paton JFR, Hart EC, et al. Investigation and Treatment of High Blood Pressure in Young People. Hypertension. 2020;75:16–22.
Acknowledgements
The authors wish to acknowledge the Hong Kong Hospital Authority for its contributions to data extraction. The computations were performed using research computing facilities offered by Information Technology Services at the University of Hong Kong.
Funding
This study was supported by Excellent Young Scientists Fund, National Natural Science Foundation of China (Principal Investigator: Eric Yuk Fai Wan; Ref No. 82222902), and the Start-up Fund of the University of Hong Kong (Principal Investigator: Eric Yuk Fai Wan). The funders have no role in the study design, data collection, data analysis, interpretation, and report drafting. The corresponding authors had full access to all the data in the study and took final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
Concept and design: KSNL, BYW, CLKL, EYFW. Acquisition of data: CLKL, EYFW. Drafting of the manuscript: KSNL, BYW. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis, or interpretation of data: BYW, EYFW. Administrative, technical, or material support: CLKL, EYFW. Supervision: EYFW.
Corresponding author
Ethics declarations
Conflict of interest
EYFW has received research grants from the Health Bureau of the Government of the Hong Kong Special Administrative Region, the Hong Kong Research Grants Council of the Government of the Hong Kong SAR, Narcotics Division, Security Bureau of the Government of the Hong Kong SAR, Social Welfare Department, Labour and Welfare Bureau of the Government of the Hong Kong SAR and National Natural Science Foundation of China, outside the submitted work. CLKL has received research grants from the Health Bureau of the Government of the Hong Kong SAR, the Hong Kong Research Grant Council, the Hong Kong College of Family Physicians, and Kerry Group Kuok Foundation, outside the submitted work. The remaining authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, K.S., Wang, B., Mak, I.L. et al. Early onset of hypertension and increased relative risks of chronic kidney disease and mortality: two population-based cohort studies in United Kingdom and Hong Kong. Hypertens Res 48, 1963–1971 (2025). https://doi.org/10.1038/s41440-025-02188-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-025-02188-x