Abstract
XPR1 is the sole protein known to transport inorganic phosphate (Pi) out of cells, a function conserved across species from yeast to mammals. Human XPR1 variants lead to cerebral calcium-phosphate deposition and primary familial brain calcification (PFBC), a hereditary neurodegenerative disorder. Here, we present the cryo-EM structure of human XPR1 in both its Pi-unbound and various Pi-bound states. XPR1 features 10 transmembrane α-helices forming an ion channel-like structure, with multiple Pi recognition sites along the channel. Pathogenic mutations in two arginine residues, which line the translocation channel, disrupt Pi transport. Molecular dynamics simulations reveal that Pi ion undergoes a stepwise transition through the sequential recognition sites during the transport process. Together with functional analyses, our results suggest that this sequential arrangement allows XPR1 to facilitate Pi ion passage via a “relay” process, and they establish a framework for the interpretation of disease-related mutations and for the development of future therapeutics.
Similar content being viewed by others
Introduction
Inorganic phosphate (Pi) is essential for life, used wildly in biomolecules synthesis, cell metabolism and energy supply. To nourish cell, humans uptake Pi through Na+-coupled Pi importers, which belong to the solute carrier 20 (SLC20) and SLC34 transporter families1,2,3,4,5. In parallel, cell exports intracellular Pi to regulate Pi homeostasis and prevent the cytotoxicity caused by Pi accumulation. Elevated Pi levels cause major disorders, with severe biochemical and clinical consequences6,7,8.
XPR1 is the only known exporter that transports Pi out of cells in humans9, expressed in all tissues10. It is a passive transporter belonging to the SLC53 family, known as SLC53A1, with no other human proteins identified within this family. Unlike active Pi importers, which utilize energy from the movements of co-translocated ions (eg., Na+ or H+) to transport Pi into cells11,12,13, XPR1 facilitates Pi efflux independently of co-translocated ions14,15,16. Loss-of-function variants of XPR1 accumulate cytosolic Pi and lead to cerebral calcium-phosphate deposition, which are associated with a genetic disease known as primary familial brain calcification (PFBC)17,18,19,20. PFBC is an adult-onset neurodegenerative disorder characterized by a wide range of clinically heterogeneous symptoms, including dystonia, parkinsonism, dementia, depression, chorea and others21. Currently, there are no targeted drugs or specific treatments available22,23. XPR1 is abundant in human platelets, where inhibiting its transport activity increases the risk of thrombosis in mouse models24. XPR1 deficiency in ovarian cancer cells causes toxic accumulation of cytosolic Pi, leading to cell death25. Dysregulation of Pi efflux could thus present a new, potential strategy for anticancer therapy16,25. Given the significance and pathomechanisms of XPR1, it presents a promising therapeutic target for diseases and cancers17,25,26,27. However, the molecular basis for this fundamental transporter remains unknown. We therefore set out to determine the cryo-electron microscopy (cryo-EM) structure of human XPR1, and to establish how it recognizes and transports Pi across cell membrane.
Results
The overall cryo-EM structure
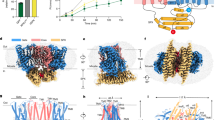
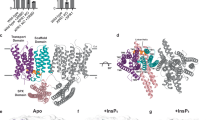
XPR1 contains a transmembrane ___domain and a cytoplasmic N-terminal SPX (named after yeast SYG1, Pho81, and human XPR1) ___domain. The transmembrane ___domain is responsible for facilitating Pi efflux, and the SPX ___domain is thought to modulate this function by binding inositol pyrophosphate (PP-InsP) stimuli9,14,28,29,30. To aid cryo-EM structure determination, we included InsP6 into the buffers used throughout the protein preparation process (Supplementary Fig. 1, and “Methods”). InsP6 is a commercially available surrogate for PP-InsPs31,32, which has been indicated to potentially limit the structural heterogeneity of SPX domains33,34. XPR1 was tagged with a C-terminal 3 × Flag tandem affinity tag and expressed in Expi293F cells. Proteins were extracted from the cells using a buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM InsP6, 1% LMNG (lauryl maltose neopentyl glycol), 0.1% CHS (cholesterol hemisuccinate) and 0.25% Soy Phospholipids. The target protein was then purified using anti-Flag G1 affinity resin and subjected to size-exclusion chromatography (SEC) on a Superose-6 Increase 10/300 column. The SEC purification buffer consisted of 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 m DTT, 1 mM InsP6, and 0.02% GDN (lglyco-diosgenin). Peak fractions were collected and pooled to ~4.5 mg ml−1 for cyro-EM grid preparation and data collection. A total of 20,420 cryo-EM micrographs were collected and processed through single-particle analysis (Supplementary Fig. 2, and “Methods”). Following series 3D classification, 1,168,388 particles were pooled, capturing millions of snapshots of the molecules in their various conformational states. We then reprocessed this particle pool into 10 classes using heterogeneous cryo-EM structure reconstruction, ensuring that each particle was assigned to only one class. Consequently, four distinct density maps of XPR1 were obtained from the final 10 reconstructions, with overall resolutions ranging from 2.9 to 3.3 Å. The highest resolution map is identified as the Pi-unbound form, while the other three maps correspond to Pi-bound forms, each displaying additional nonprotein densities within a membrane-spanning pore of the XPR1 transmembrane ___domain (Discussed later). Upon thorough examination, we found that the other 6 class maps either exhibit medium resolutions (ranging from 3.2 Å to 4.1 Å) or display no significant Pi-like densities within the protein. We, therefore, did not perform analyses related to these 6 classes in our manuscript. The EM density of SPX ___domain was observed to be poor (Supplementary Fig. 3a), probably owing to its mobility. By contrast, the density map of the transmembrane ___domain was well resolved (Supplementary Fig. 3a), enabling the construction of an atomic model (Supplementary Fig. 3b, c, and Supplementary Table 1). XPR1 forms dimers in the micelles (Supplementary Fig. 3a, b), with transmembrane domains loosely contacted, indicating that they may function as independent units.
The transmembrane ___domain of XPR1 contains 10 transmembrane α-helices (TM1-10) and folds into two structurally distinct sub-domains, with N and C termini on the intracellular side (Fig. 1). We refer to the N-terminal portion as N ___domain, that is formed by TM1-TM5 and a short amphipathic helix (AH) lying parallel to the membrane. The C-terminal portion harbors the conserved EXS (named for homologous regions found in yeast ERD1 and SYG1 and human XPR1) ___domain35,36 (Supplementary Fig. 4), that is made up of TM6-TM10. The EXS ___domain associates with TM5, creating a pore that spans across the membrane (Fig. 1a). Notably, the TM9 bends and positions TM9b close to the central pore axis on the extracellular side (Fig. 1a), suggesting its potential role in Pi export. This experimental structure differs from the predicted model of AlphaFold237 (Supplementary Fig. 5), and a 3-dimensional structural homology search with the program DALI38 found no similar known structures, indicating a specific mechanism for phosphate recognition and transport in XPR1.
a Cartoon representation of the structure. The N ___domain and EXS ___domain are colored in magenta and blue, respectively. TM9b is colored in yellow. b Schematic topology diagram of the structure. The gray background indicates the membrane bilayer. The N-terminal SPX ___domain of XPR1 is invisible in the determined cryo-EM structure.
The structure basis for phosphate recognition and transport
To gain insight into the pathway for Pi ion translocation, we conducted CAVER39 analysis for the resolved structure. The analysis revealed a continuous, solvent-accessible pathway within the XPR1 transmembrane ___domain, featuring an ion channel-like architecture (Fig. 2a, b, and Supplementary Fig. 6a). The narrowest point of this channel has a radius of ~1.2 Å (Fig. 2b), which exceeds the water access limit (1 Å)40, suggesting its permeability to solvents and potential for Pi ion conduction. As this channel is smaller than the ionic radius of Pi (2.38 Å), the observed conformation is presumably a partially open state. The intracellular entrance of the translocation channel is formed by TM10, TM6b, TM7, TM5a, and TM8 (Fig. 2a, Supplementary Fig. 6a). Owing to the bending of TM9, the channel is curved and created by TM9b, TM10, TM6a, TM5b and the tip of TM2 on the extracellular side. The channel is primarily lined with polar residues, characterized by a positively charged interior wall (Fig. 2a, Supplementary Fig. 6b), which presumably facilitates the permeation of anionic Pi ions. The narrow regions along the ion permeation pathway is constricted mainly by residues D398, Y483, N401, R570, S404, and W573 (Fig. 2b), implying their roles in Pi transport.
a Channel-like architecture. Gray surface represents the channel path within XPR1 transmembrane ___domain. Transmembrane α-helices forming the channel are indicated, and channel-lining residues are shown as sticks. b Pore radius along the channel. The gray dashed line indicates a radius of 1 Å for water access limit. c Structures superposition. The transmembrane ___domain structures of Pi-unbound XPR1, Pi-bound XPR1Pi1, Pi-bound XPR1Pi2, and Pi-bound XPR1Pi3 are colored in gray, cyan, pink, and green, respectively. Only the channel-forming transmembrane α-helices are represented for clarity. Pi ion in each structure is shown as sticks. d–f Pi recognition sties 1, 2, and 3, observed in the structures of XPR1Pi1, XPR1Pi2, XPR1Pi3, respectively. The black dashed lines indicate plausible interactions between the Pi ion and the protein, while the red dashed lines represent the interactions that may indirectly contribute to Pi coordination. The Pi-recognizing residues at sites 1 and 2 largely overlap but adopt different binding models. g, h Activity assay. The Pi transport activity is determined by measuring phosphate uptake into liposomes containing wild-type and variant forms of XPR1. Activity of PFBC families variants, R459C, R570C and R570L, are highlighted in red. The time-course activity (g) was collected at external Pi concentration of 500 μM, with transport reaction times of 3, 6, 9, 12, 18, and 24 min. The data presented of R570C and R570L are the average of 3 independent assays, while the others are averaged from 4 independent assays, each with technical triplicates. The error bars indicates the SD. The single-point activity (h) was collected at the external Pi concentration of 500 μM, with a transport reaction time of 6 min. The data presented are the average of 3–4 independent assays, each with technical triplicates. The error bars indicates the SD.
In parallel to the above-resolved XPR1 transmembrane ___domain structure (Pi-unbound XPR1), our structural heterogeneous analysis has reconstructed three additional cryo-EM maps with resolutions of 3.1–3.3 Å (Supplementary Fig. 2, and “Methods”). These reconstructed maps revealed extra nonprotein densities positioned at various locations inside the translocation channel. These clear densities were putatively modeled with Pi ions and are well-positioned by surrounding residues within the channel (Fig. 2c–f, Supplementary Fig. 7). As no phosphate was added during our sample preparation, we assume that the observed Pi ions were copurified with XPR1 endogenously. Moving in the intracellular-to-extracellular direction, the three structures are denoted as XPR1Pi1, XPR1Pi2 and XPR1Pi3, based on the positions of Pi ions lined along the channel (Fig. 2c). Given that the three Pi ions are bound at different positions in the channel, we refer to the three sites as Pi recognition site 1, 2 and 3, respectively. The three structures are also dimeric and their protomers show overall structural similarity to that of Pi-unbound XPR1, with RMSD of 0.26–0.62 Å. This suggests that Pi binding does not induce large conformational changes. The aqueous pore within XPR1 transmembrane ___domain (Fig. 2b), together with aligned Pi ions along the permeation pathway (Fig. 2c), suggests that XPR1 may facilitate the passive diffusion of Pi ion through this channel without requiring large conformational changes, as observed in Pi importers. Pi importers allow only one side to be solvent-associable at a time. They exhibit an outward-open conformation for Pi uptake from the extracellular side and subsequently undergo large conformational changes to adopt an inward-open conformation, allowing for Pi release into cytosol11,12,13. This conformational transition is driven by the energy derived from movements of other co-translocated ions (e.g., Na+ or protons) down their concentration gradients11,12,13. By contrast, the Pi export capability of XPR1 is independent of pH gradient across the cell membrane14,15, and no co-translocated ions have been found16. These previously observed phenomena are now supported and aligned with the channel-like architecture of XPR1.
Our structures reveal stepwise states of Pi ion transitions within the translocation channel, providing a framework for understanding how XPR1 recognizes and transports phosphate. The translocation channel is lined by a set of polar and charged residues, which provide sites for the recognition of ionic Pi (Fig. 2a, Supplementary Fig. 6b). At Pi recognition site 1, the structure of XPR1Pi1 reveals that the Pi ion (Pi1) is primarily recognized through interactions with the side chains of D398, K482, Y483, D529, D533, R570, R604, and the indole N atom of the W607 (Fig. 2d). These interactions include hydrogen bonds and salt bridges between the Pi1 ion and the protein, with details listed in Supplementary Table 2. Additionally, R459 and N401 on the periphery likely orient the side chains of D398 and Y483, facilitating their coordination of Pi1 at the binding site. At Pi recognition site 2, the Pi-recognizing residues mainly overlap with those at Pi recognition site 1, but they adopt a different binding model. As the Pi ion transitions to site 2, it (Pi2) disengages from D529, D533, and W607, forming new interactions with R603 and N401 (Fig. 2e, Supplementary Table 2). During this transition, the peripheral E600 rotates and establishes salt bridges with R603, positioning R603 for Pi2 coordination. When the Pi ion moves upward (intracellular-to-extracellular direction), the XPR1Pi3 structure captures a state where the Pi ion (Pi3) is coordinated at recognition site 3 (Fig. 2f). At this site, the Pi3 ion dissociates from R570 and forms direct interactions with the side chains of N401, S404, Q452, and W573. Additionally, R570 and I577 contribute indirectly to this recognition site by forming cation-π and CH-π interactions with W573, respectively. These observed Pi-recognizing residues are largely conserved among EXS ___domain-containing proteins across different species (Supplementary Fig. 4), suggesting functional and mechanistic conservations.
To gain functional support for the above-identified key residues in Pi transport, we reconstituted XPR1 into liposomes and performed Pi transport assays (Methods). Results showed that the reconstituted XPR1 exhibited Pi transport activity and transported Pi into the proteoliposomes in an external Pi concentration-dependent manner (Supplementary Fig. 8), as well as a time-dependent manner (Fig. 2g). The Pi transport activity correlates linearly to the external Pi concentration up to 1 mM in the established assays (Supplementary Fig. 8), implying that XPR1 may operate as a low-affinity Pi transporter with a Km in millimolar range. By substituting the Pi-recognizing residues D398, K482, D533, R604, R603, N401, and Q452 with alanine, and Y483 and W607 with phenylalanine, we could purify the Y483F, R603A, N401A, and Q452A in sufficient quantities to perform the proteoliposome-based transport assays. Time-course results showed that XPR1 carrying Y483F, R603A, N401A or Q452A substitution has impaired Pi transport activity (Fig. 2g), revealing crucial roles of these residues in Pi transport. Moreover, consistent with the structural role of the E600 side chain in positioning R603 at Pi recognition site 2 (Fig. 2e), replacing E600 with alanine resulted in a reduced Pi transport kinetic of XPR1 (Fig. 2g). At Pi recognition site 3, the polar NE1 atom of W573 and its bulky indole ring likely cooperate in Pi coordination (Fig. 2f). Supporting this, substituting the side chain of W573 with polar or hydrophobic groups such as W573N, W573Y, W573A and W573L reduced the Pi transport activity of XPR1 (Fig. 2h). Moreover, we employed microscale thermophoresis (MST) measurements to assess the Pi binding affinity of XPR1 and its mutants. The results indicates that the detergent-solubilized XPR1 binds Pi with a Kd of 334.1 ± 79.3 μM (Supplementary Fig. 9). Substitutions of those Pi-recognizing residues led to reduced Pi binding affinity (Supplementary Fig. 9), which aligns with their impaired Pi transport activities as revealed by proteoliposome-based assays (Fig. 2g, h).
Additionally, we evaluated the significance of these Pi-recognizing residues by performing Pi transport assays in a heterologous yeast system. We utilized a yeast mutant strain (YP100) lacking Pi transporters, which cannot grow on YNB media41. Previous study found that transformation of this mutant yeast with rice OsPHO1;2, a functional homolog of human XPR1, can rescue the yeast growth phenotype15. Similarly, we observed that complementation with XPR1 also restored yeast growth (Supplementary Fig. 10). In contrast, yeast strains complemented with XPR1 mutants, including N401A, Q452A, Y483F, W573A, W573N, W573L, W573Y, E600A, and R603A, exhibited impaired growth (Supplementary Fig. 9). These findings corroborate our proteoliposome-based assays results (Fig. 2g, h), underscoring the crucial roles of these residues in Pi transport.
Clinically linked residues in XPR1 function in phosphate recognition and transport
Autosomal dominant XPR1 mutations are associated with PFBC17,19,20, including the R459C, R570C, and R570L missense variants18,23,42,43,44. Our structures reveal that R459 contributes to establish the Pi binding network at recognition sites 1 and 2, while R570 plays a direct role in Pi recognition during its translocation (Fig. 2d–f). Substituting the side chains of R459 and R570 might perturb Pi binding and potentially impair XPR1 activity. Indeed, we find that the PFBC families variants, R459C, R570C, and R570L, exhibit a significant reduction in Pi transport activity (Fig. 2g). The impaired function of these mutants is consistent with their reduced Pi binding affinity (Supplementary Fig. 9), and is also evident in the heterologous yeast assays (Supplementary Fig. 10). This Pi transport deficiency observed in these hereditary XPR1 variants aligns with clinical findings of accumulated cytosolic Pi and cerebral calcium-phosphate deposition in PFBC patients. Therefore, our structure and function analyses provide mechanistic insights into the correlation between patient mutations and XPR1 function.
Dynamic insights into phosphate transport mechanism
To gain dynamic insights into the channel-like transport mechanism of XPR1, we performed two independent 1000-ns all-atom molecular dynamics (MD) simulations using the Pi-unbound XPR1 cryo-EM structure (Methods). These simulations aimed to assess the stability of the structure and evaluate the permeation properties of the channel-like pore. The simulations results showed that the overall structure remained stable, as reflected by the relatively small root mean square deviations (RMSD) of the transmembrane helices, despite fluctuations in the loop regions (Supplementary Fig. 11a–c). Throughout the simulations, the transmembrane pore consistently exhibited hydration (Supplementary Fig. 11d), suggesting its potential to facilitate Pi ion permeation.
To further investigate the Pi transport process, we performed MD simulations on the Pi-bound XPR1 (XPR1Pi1) structure under an applied electric field (“Methods”). Following the simulations, we observed an export event of Pi ion through the channel, where the Pi ion dissociated from the recognition site 1, moved upward, and released into the extracellular solution (Supplementary Video 1). Minor structural changes were observed in XPR1 during the transport process, further supporting its channel-like properties (Supplementary Fig. 12a). The simulations also showed that the transmembrane pore remained hydrated, similar to the Pi-unbound form, allowing the Pi ion to remain solvated and diffuse freely within the channel (Fig. 3a). Interestingly, the Pi ion traversed the entire length of the channel, following a stepwise mechanism that involved three distinct binding sites (Fig. 3b, Supplementary Fig. 12b, and Supplementary Video 1). These binding sites aligned well with the conformations observed in our cryo-EM structures, suggesting they likely represent stable intermediates in the transport process.
a Side-cut view of the channel, showing the average water density from the simulation as a gray transparent surface. The Pi ion positions sampled across all simulation frames are depicted as red spheres, illustrating its diffusion within the translocation channel. The distance (Distz) between the Pi ion and the center of mass of the phosphate head groups of lipids in the inner leaflet along the membrane’s normal direction (Z-axis) is defined and labeled on the right. b Movement of the Pi ion through the channel, monitored by Distz in the simulations. The Pi ion is released into the extracellular solution at ~350 ns of simulation time. The gray dashed lines represent the positions of Pi ions as observed in the cryo-EM structures of XPR1Pi1, XPR1Pi2, and XPR1Pi3. c Comparison between cryo-EM structures and conformational snapshots from MD simulations. The Pi recognition sites 2 and 3 conformations in the XPR1Pi2 and XPR1Pi3 structures are colored pink and green, respectively, while similar conformations captured at ~138 ns and ~333 ns of simulation time are shown in gray. d Trajectories of Pi-residue contacts to show representative interactions between the Pi ion and specific residues during its transport process.
Our cryo-EM structures provide insights into the mechanism by which the Pi ion transitions from the site 1 to site 3 (Fig. 2d–f). Specifically, as the Pi ion moves upward, it first dissociates from residues D529, D533 and W607 at site 1 (Supplementary Fig. 12c), then forms a new interaction with R603 at site 2 before transitioning to the site 3, where it is further recognized by residues S404, Q452 and W573 (Supplementary Fig. 12c). Supporting this mechanistic pathway, MD simulations revealed that the Pi ion undergoes a stepwise transition through D529, R603, and Q452, corresponding to recognition sites 1, 2 and 3, respectively (Supplementary Fig. 12d). Notably, despite beginning with the XPR1Pi1 structure, the simulations sampled conformations similar to those experimentally resolved structures of XPR1Pi2 and XPR1Pi3 (Fig. 3c). Throughout the simulations, the movement of Pi ion was facilitated by orchestrated interactions with residues from recognition site 1 to site 3 (Fig. 3d). Moreover, as the Pi ion disengaged from residues in site 3 (N401, S404, and Q452), it interacted with Q576, R448 and R270 (Fig. 3d) which are positioned above the site 3 and near the channel exit (Fig. 2a). These findings suggest that these residues may facilitate the Pi ion’s exit from the channel. Consistent with this, XPR1 mutants Q576A, R448A, and R270A exhibit reduced Pi transport activity (Fig. 2g, Supplementary Figs. 9, 10). Together, these observations reinforce our structural findings of XPR1 and enhance the understanding of the channel-like transport mechanism. We propose that XPR1 likely employs a “relay” process to facilitate Pi ion passage through the channel, with sequential Pi recognition sites arranged along its pathway.
Discussion
Elevated levels of cytosolic phosphate are cytotoxic due to its presence as a potent metal chelator and a pervasive inhibitor of cellular enzymes. XPR1 and its evolutionarily conserved orthologues are the proteins that transport cytosolic phosphate out of cells9,15,45,46,47. A very recent study discovered that the XPR1 orthologue in fruit fly (Pxo) lowers cellular phosphate levels, by generating a newly defined phosphate-storing organelle (PXo body) and transporting cytosolic phosphate into the PXo body48. These transporters belong to the solely member of the structurally uncharacterized SLC53 family. Here, the experimental structure of XPR1 transmembrane ___domain, together with its multiple functional states bound to phosphate, provides the snapshots for understanding the structure-function relationships of SLC53 family transporters.
XPR1 mutations are linked to PFBC neurodegenerative disorder for which there are currently no targeting drugs and specific treatments available17,21,22,23. Lack of structural and related functional understanding limits patient mutation interpretation to disease mechanism. In this study, our structure and function analyses reveal that the pathogenic mutation residues (R459 and R570) line the phosphate translocation channel and act in phosphate recognition and transport. Notably, the MD simulations indicate that residue R570 interacts with Pi ion throughout the entire transport process (Fig. 3d), underscoring the significance of R570. These mechanistic understanding might provide an opportunity for targeting XPR1 in the development of future therapeutics.
XPR1 exhibits a channel-like architecture that facilitates phosphate efflux, distinguishing it from phosphate importers that use an alternating access mechanism for phosphate uptake11,12,49,50. This variety in mechanisms for balancing cellular phosphate levels offers cell regulatory layers. The activity of phosphate importers is regulated by the concentration gradient of other co-translocated ions (e.g., Na+ or proton)11,12,13,16, but no co-translocated ions are required for XPR1 function14,15,16. Instead, XPR1 harbors an additional cytoplasmic SPX ___domain, which modulates XPR1 function by sensing PP-InsP nutrient messengers9,14,28,29,30. PP-InsPs have emerged as cellular high-Pi signals in regulating phosphate homeostasis31,32,33,51,52. The limited cryo-EM density observed for the SPX ___domain in our reconstructed map suggests its mobility. This mobile nature might facilitate PP-InsPs targeting and regulation.
During the revision of our manuscript, Yan et al.53 and Lu et al.54 independently reported cryo-EM studies of XPR1 structures, also revealing the channel-like architecture and mechanism for Pi recognition and transport. Yan et al.53 determined the Pi-bound TMD structure in closed and open conformations. Notably, the site 2 in their closed conformation overlaps with the recognition site 2 observed in our XPR1Pi2 structure. While Yan et al. identified two additional Pi binding sites at the intracellular entrance and extracellular exit of the Pi translocation channel, we observed further binding sites deeper within the channel (Pi recognition site 1 and 3). Collectively, all these identified Pi binding sites are distributed throughout the channel, supporting the channel-like transport mechanism. Yan et al.53 and Lu et al.54 determined InsP6- or PP-InsP7- bound XPR1 structures with TMD in a closed conformation, providing further understanding of PP-InsPs regulation of XPR1. It is proposed that the TMD channel operates independently of the SPX ___domain and PP-InsPs sensing53. Specifically, movements of the PP-InsPs-linked SPX ___domain regulate the entry of intracellular Pi into the channel, while the gating for the outward movement is driven by the intracellular Pi concentration across the cell membrane and the resting membrane potential. This is corroborated by our MD simulations of XPR1Pi1, which indicate that the export event of Pi ion through the channel occurs only under an applied external electric field, with no transport events observed in the absence of this field. Although our MD simulations indicate a transit time of about 350 ns for Pi through the channel (Fig. 3b), the observed Pi transport rate is only ~3 pmol Pi/pmol protein/minute (equivalent to 33 pmol Pi/μg protein/minute) under a 500 μM Pi concentration gradient, as measured by the reconstituted proteoliposome-based assays (Fig. 2g). This transport rate is largely slower than that of a typical ion channel, which can transport millions of ions per second. We speculate that this low transport activity of XPR1 may be attributed to the lack of a membrane potential in the reconstituted system. In Lu et al.’s study54, their single-channel electrophysiological analyses characterized rapid conductance events of XPR1, ranging between 10 and 15 ps, under an applied −80 mV potential and a high concentration of 10 mM InsP6 stimulus. Further studies, in a reconstituted system, are needed to understand the intriguing relationship between transport activity and membrane potential. Together, our work, in conjunction with the published studies, provides a complementary understanding of the structural mechanisms underlying Pi recognition and transport by human XPR1.
Methods
Protein expression and purification
Human XPR1 DNA was subcloned into a pMlink vector encompassing a C-terminal 3×Flag tandem affinity tag. Point mutations were introduced into XPR1 genes by overlapping PCR and were verified by DNA sequencing. Proteins were expressed in Expi293FTM cells (Invitrogen) by transient transfection. Cells grown in Union-293 media (Union-Biotech, Shanghai) were transfected with linear polyethyleneimine (PEI) (Polysciences) at a cell density of 2.0 × 106 cells per ml−1. The transfected cells were cultured for another 60 h before harvesting.
To prepare the cryo-EM sample, cultured cells were collected and resuspended in the TBS buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM InsP6 (a commercially available surrogate for PP-InsPs32), 1% LMNG (Anatrace), 0.1% CHS (Anatrace) and 0.25% Soy Phospholipids (Sigma). The extraction were performed at 4 °C for 1.5 h, and the resulting solution was centrifuged at 23,000 × g for 40 min. The supernatant was collected and incubated with anti-Flag G1 affinity resin (Genscript) at 4 °C for 40 min, further rinsed with 30 bed volumes of wash (W1) buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM InsP6 and 0.02% GDN (Anatrace), and eluted by W1 buffer supplemented with 250 μg ml−1 Flag peptide (Genscript). The eluent was concentrated and further purified by size-exclusion chromatography (Superose-6 Increase 10/300 column, GE Healthcare) using a buffer containing 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM DTT, 1 mM InsP6 and 0.02% GDN. The peak fractions of XPR1 were collected and pooled to ~4.5 mg ml−1 for cryo-EM grid preparation.
Sample used for the liposome-based transport assays were extracted from cultured cells by a buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl and 1% n-Dodecyl-β-D-Maltopyranoside (DDM). Target proteins were further purified using anti-Flag G1 affinity resin and Superose-6 Increase 10/300 column in tandem, and prepared in the buffer containing 25 mM Hepes-Tris (pH 7.4), 150 mM NaCl, 2 mM DTT, and 0.02% DDM for further proteoliposomes reconstitution.
Cryo-EM grid preparation and data collection
3.5 μl aliquots of the purified protein was dispensed onto glow discharged holey carbon grid (Quantifoil Cu R1.2/1.3, 300 mesh). The gird was blotted with a Vitrobot Mark IV (ThemoFisher Scientific) using 3.5 s blotting time with 100% humidity at 8 °C, and was plunge-frozen in liquid ethane. The cryo-grid was transferred to 300 kV Titan Krios electron microscopes (Thermo Fisher) equipped with a GIF Quantum energy filter (slit width 20 eV) and a Gatan K3 Summit detector. EPU software (v2.9) was used for fully automated data collection. Micrographs were recorded in the super-resolution mode with a magnification of 81,000×. Each micrograph stack, which contains 32 frames, was exposed for 3.5 s with a total electron dose of 50 e−/Å2. MotionCor2 (v1.4.7)55 was used to perform beam-induced motion correction on cryo-EM images with binning factor of 2, resulting in a pixel size of 1.07 Å. The defocus value of each image was set to −1.2 to −2.2 μm and estimated by CTFFIND4 (v4.1.14)56.
Cryo-EM data processing
A diagram of the procedures for data processing was described in Supplementary Fig. 2. For the structure determination, 19,877 micrographs were manually selected from two datasets of 20,420 micrographs. A total of 24,071,915 particles were selected and extracted for 2D classification, out of which 23,126,090 particles were selected for 3D classification. After several rounds of 3D classification using the “multi-reference” approach, the 1,168,388 particles with the best class were re-extracted to their original size for 3D refinement, resulting in a cryo-EM density map with an overall resolution of 2.9 Å. This map clearly displays the transmembrane ___domain of XPR1, which forms dimers in the micelles. To preserve asymmetric features, C1 symmetry was employed during the 3D refinement. To search for and determine potential phosphate densities within XPR1 transmembrane ___domain, we further conducted structural heterogeneity analysis on these pooled particles using 3D Classification (BETA) in cyoSPARC and cryoDRGN (v3.4.0)57,58. These particles were mandatorily classified into 10 classes, with each particle belonging to only one class. The target resolution for each reconstruction was set at 3 Å, ensuring that each class was independent. Subsequently, a meticulous examination of the 3D-refined cryo-EM maps was conducted, resulting in the acquisition of a 2.9-Å cryo-EM map for XPR1 in the Pi-unbound form and three maps for the Pi-bound forms, with resolutions of 3.1, 3.1, and 3.3 Å, respectively. CryoSPARC (v4.5.1)57 and RELION (v5.0)59 were used for 2D classification, 3D classification and 3D refinement. Local resolution variations of the maps were estimated using Resmap (v1.1.4)60.
Model building and refinement
The initial model of XPR1 was predicted from the Alphafold237. We employed the ChimeraX software to dock this predicted model into the reconstructed cryo-EM map. The model was manually refined through iterative rounds of adjustments using COOT61. The residues of XPR1 transmembrane α-helices can be effectively constructed in the model. The phospholipid-like densities are assigned as phosphatidylcholine. Nonprotein densities were observed in one protomer of each dimer in the XPR1Pi1 and XPR1Pi2 cryo-EM maps, and in both protomers of the dimer in the XPR1Pi3 cryo-EM map. These densities were modeled with Pi ions. The obtained model was refined against the map using PHENIX62 in real space with secondary and geometry restrains. Model quality assessments were conducted via Molprobity scores63 and Ramachandran plots. Structural Figures were generated using ChimeraX (v1.6.1) and Pymol (v2.4.1).
Transport assay
Liposomes (10 mg/mL) were prepared with E. coli total extract (Avanti Polar Lipids) in a reconstitution buffer containing 10 mM Hepes-Tris (pH 7.4) and 100 mM KCl. Preformed liposomes were dissolved with 1.3 % (w/v) DDM and mixed with purified XPR1 or variants in a protein-to-lipid ratio of 1:100 (w/w). Following incubation at 4 °C for 1.5 h, the DDM was removed by 3 additions of SM-2 bio-beads (Bio-Rad), incubated for 2 h/2 h/overnight, respectively. Prior to the start of the transport assay, the proteoliposomes were extruded using polycarbonate filter with a pore size of 200 nm (Whatman). 15 μl proteoliposomes containing 0.2–0.5 μg protein were diluted into 80 μl reconstitution buffer. Pi transport reactions were initiated by adding a KPi mixture at given concentration, which contains non-labeled KH2PO4 and [32P] KH2PO4 (3.7 MBq/ μmol; PerkinElmer) in a molar ratio of 29:1–3:1. The assays were performed at 37 °C, and terminated at given times by diluting tenfold with ice-cold stop buffer (10 mM Hepes-Tris, pH 7.4, 100 mM KCl and 5 mM non-labeled KH2PO4), followed by rapid filtration through nitrocellulose membrane (Millipore, 0.22 μm Triton-free MCE). The filters were subsequently washed with 2 × 5 ml ice-cold stop buffer, placed in 5 mL Optiphase HiSafe 3 scintillation fluid, and counted after 14 h. Background was defined as the counts of parallel transport assays that were terminated at the beginning of the reaction. After subtracting the background, the amount of phosphate transported inside the proteoliposomes was quantified by comparing it to a standard curve of 32P KH2PO4. This standard curve was established by diluting sole 32P KH2PO4 in water and recording the corresponding radioactive counts. It thus provided a quantitative relationship between radioactive counts and the amount of 32P, which was used to quantify the amount of Pi taken up during our transport reactions. The protein contained in proteoliposomes were resolved by SDS-PAGE and quantified using ImageJ. The Pi transport activity is determined by measuring phosphate uptake into proteoliposomes containing proteins (pmol Pi/μg protein). The assays were independently performed a minimal of three times, each with technical triplicates, to generate an overall mean and s.d.
Microscale thermophoresis measurement
The Pi binding affinity of XPR1 and its mutants were measured by microscale thermophoresis using a Monolith NT.115 instrument (Nano Temper Techologies, Germany). Protein were prepared through size exclusion chromatography in a SEC buffer containing 25 mM Tris pH 8.0, 150 mM NaCl and 0.02% DDM. The protein was labeled by mixing it with FITC (Fluorescein-5-Maleimide) dye at a molar ratio of 1:4. After 1.5 h incubation at room temperature, the excess dye was removed by using a desalting column. A volume of 10 μL of ~100 nM dye-labeled XPR1 was mixed with 10 μL ligand (varying concentrations between 30.52 μM and 1 M) in the SEC buffer. The XPR1-ligand mixture was loaded into capillaries (Nano Temper Technologies), and thermophoresis was measured at 25 °C with an excitation power of 20% and an MST power of medium. Data from at least three independent measurements were combined and analyzed using MO Affinity Analysis software (Nano Temper Technologies), and fitted to a single binding site model.
Yeast complementation
Corresponding genes were separately constructed into a PRS416-ADH vector and transformed into the yeast Pi transport-deficient mutant YP100 strain41. Transformants were selected on synthetic complete (SC-uracil) yeast medium (containing 2% galactose, 0.67% yeast nitrogen base without amino acids, 0.2% appropriate amino acids, and 2% agar) at pH 5.4. The medium was incubated at 28 °C for 2–3 days, and transformants were verified by PCR. 2× YPD medium was used for transformants to cultivate. Mid-exponential phase cells were collected, washed two times with YNB (without phosphate) and resuspended to OD600 = 1 in water. Equal volumes of 5-fold serial dilutions were spotted on YNB medium (supplemented with 2% glucose, 7.5 mM KH2PO4, 2% agar, and 7.5% amino acids mix, deficient uracil) at pH 5.4. Plates were incubated at 28 °C for 7 days to observe yeast growth.
Molecular dynamic simulations
Atomistic models of XPR1 transmembrane ___domain were constructed using the structures of Pi-unbound XPR1 and Pi-bound XPR1Pi1. The missing loop (amino acids 432–445) was modeled using the AlphaFold2 prediction as a template. These models were then embedded in a POPC lipid bilayer and solvated in a cubic water box containing 0.15 M NaCl. The lipids identified in the cryo-EM density maps were also modeled as POPC. The dimensions of the box were 8.0 nm × 8.0 nm × 8.2 nm in the x, y, and z directions, respectively, totaling ~47,000 atoms. The transmembrane region was aligned in the lipid bilayer using the OPM (Orientations of Proteins in Membranes) webserver. Construction of the systems was facilitated by the CHARMM-GUI webserver64, followed by energy minimization using the steepest descent algorithm and a six-step equilibration process with gradual removal of position constraints. Production runs under semi-isothermal-isobaric (NPT) conditions were conducted using the CHARMM36m force field65 for proteins and lipids, along with the TIP3P model for water. The Pi ion in the XPR1Pi1 structure was modeled as H2PO4-. The protonation states of residues in the Pi binding sites were assigned according to their pKa values predicted by PROPKA3.166 and further justified by examining the local environment. During the MD simulations, temperature was maintained at 310 K with a Nosé–Hoover thermostat and a coupling constant of 1 ps, and pressure was kept at 1.0 bar using the Parrinello–Rahman barostat and a time coupling constant of 5 ps. A switch function with a starting distance of 1.0 nm was employed for a van der Waals cut-off of 1.2 nm. Short-range electrostatic interactions were also truncated at 1.2 nm, while long-range electrostatic interactions were computed via the particle mesh Ewald decomposition algorithm with a 0.12-nm mesh spacing. For each system, two independent 1000 ns MD simulations were conducted using the GPU-accelerated version of Gromacs 2024.267.
To accelerate Pi transport and enable the observation of transport events within feasible simulation timescales, a constant electrostatic field along the membrane normal was applied to simulate a transmembrane voltage of ~400 mV. Although this voltage is higher than typical physiological conditions (usually around −70 to −90 mV in cells), it is widely used in simulations of ion transporters. This value is considered high enough to drive the transport process effectively while remaining within a range that ensures the robustness and relevance of the simulation results. Trajectory analysis was performed using Gromacs gmx tools and PLUMED 2.8.268. The water density map was analyzed with GROmaps69.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support this study are available from the corresponding authors upon request. The EM maps and atomic models of Pi-unbound XPR1, Pi-bound XPR1Pi1, XPR1Pi2 and XPR1Pi3 have been deposited in the Electron Microscopy Data Bank and Protein Data Bank, with the accession numbers EMD-61138, EMD-61139, EMD-61140 and EMD-61141, and 9J4X, 9J51, 9J52 and 9J53, respectively. The source data underlying Fig. 1g, h and Supplementary Figs. 1, 8, and 9 are provided as a Source Data file. The files relating to the MD simulations can be found at Github [https://github.com/yongwangCPH/papers/tree/main/2024/XPR1]. Source data are provided with this paper.
References
Kavanaugh, M. P. et al. Cell-surface receptors for gibbon ape leukemia-virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA 91, 7071–7075 (1994).
Olah, Z., Lehel, C., Anderson, W. B., Eiden, M. V. & Wilson, C. A. The cellular receptor for gibbon ape leukemia-virus is a novel high-affinity sodium-dependent phosphate transporter. J. Biol. Chem. 269, 25426–25431 (1994).
Hilfiker, H. et al. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc. Natl. Acad. Sci. USA 95, 14564–14569 (1998).
Murer, H., Hernando, N., Forster, I. & Biber, J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol. Rev. 80, 1373–1409 (2000).
Forster, I. C., Hernando, N., Biber, J. & Murer, H. Phosphate transporters of the SLC20 and SLC34 families. Mol. Asp. Med. 34, 386–395 (2013).
Chande, S. & Bergwitz, C. Role of phosphate sensing in bone and mineral metabolism. Nat. Rev. Endocrinol. 14, 637–655 (2018).
Prie, D. & Friedlander, G. Genetic disorders of renal phosphate transport. N. Engl. J. Med. 362, 2399–2409 (2010).
Manghat, P., Sodi, R. & Swaminathan, R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 51, 631–656 (2014).
Giovannini, D., Touhami, J., Charnet, P., Sitbon, M. & Battini, J. L. Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep. 3, 1866–1873 (2013).
Tailor, C. S., Nouri, A., Lee, C. G., Kozak, C. & Kabat, D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. USA 96, 927–932 (1999).
Pedersen, B. P. et al. Crystal structure of a eukaryotic phosphate transporter. Nature 496, 533–536 (2013).
Tsai, J. Y. et al. Structure of the sodium-dependent phosphate transporter reveals insights into human solute carrier SLC20. Sci. Adv. 6, eabb4024 (2020).
Liu, Y. et al. Key computational findings reveal proton transfer as driving the functional cycle in the phosphate transporter PiPT. Proc. Natl. Acad. Sci. USA 118, e2101932118 (2021).
Lopez-Sanchez, U. et al. Interplay between primary familial brain calcification-associated SLC20A2 and XPR1 phosphate transporters requires inositol polyphosphates for control of cellular phosphate homeostasis. J. Biol. Chem. 295, 9366–9378 (2020).
Ma, B. et al. A plasma membrane transporter coordinates phosphate reallocation and grain filling in cereals. Nat. Genet. 53, 906–915 (2021).
Jennings, M. L. Role of transporters in regulating mammalian intracellular inorganic phosphate. Front. Pharmacol. 14, 1163442 (2023).
Legati, A. et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 47, 579–581 (2015).
Balck, A. et al. Genotype-phenotype relations in primary familial brain calcification: systematic MDSGene review. Mov. Disord. 36, 2468–2480 (2021).
Yao, X. P. et al. Analysis of gene expression and functional characterization of XPR1: a pathogenic gene for primary familial brain calcification. Cell Tissue Res. 370, 267–273 (2017).
Anheim, M. et al. XPR1 mutations are a rare cause of primary familial brain calcification. J. Neurol. 263, 1559–1564 (2016).
Sobrido, M. J., Coppola, G., Oliveira, J., Hopfer, S. & Geschwind, D. H. Primary familial brain calcification. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1421/ (2014).
Xu, X. et al. The pathology of primary familial brain calcification: implications for treatment. Neurosci. Bull. 39, 659–674 (2023).
Ramos, E. M. et al. Primary brain calcification: an international study reporting novel variants and associated phenotypes. Eur. J. Hum. Genet. 26, 1462–1477 (2018).
Mailer, R. K. et al. Xenotropic and polytropic retrovirus receptor 1 regulates procoagulant platelet polyphosphate. Blood 137, 1392–1405 (2021).
Bondeson, D. P. et al. Phosphate dysregulation via the XPR1-KIDINS220 protein complex is a therapeutic vulnerability in ovarian cancer. Nat. Cancer 3, 681–695 (2022).
Quintans, B., Oliveira, J. & Sobrido, M.-J. Primary familial brain calcifications. Handb. Clin. Neurol. 147, 307–317 (2018).
Akasu-Nagayoshi, Y. et al. PHOSPHATE exporter XPR1/SLC53A1 is required for the tumorigenicity of epithelial ovarian cancer. Cancer Sci. 113, 2034–2043 (2022).
Li, X. Y. et al. Control of XPR1-dependent cellular phosphate efflux by InsP(8) is an exemplar for functionally-exclusive inositol pyrophosphate signaling. Proc. Natl. Acad. Sci. USA 117, 3568–3574 (2020).
Moritoh, Y. et al. The enzymatic activity of inositol hexakisphosphate kinase controls circulating phosphate in mammals. Nat. Commun. 12, 4847 (2021).
Li, X. Y. et al. Homeostatic coordination of cellular phosphate uptake and efflux requires an organelle-based receptor for the inositol pyrophosphate IP8. Cell Rep. 43, 114316 (2024).
Wild, R. et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352, 986–990 (2016).
Guan, Z. et al. The cytoplasmic synthesis and coupled membrane translocation of eukaryotic polyphosphate by signal-activated VTC complex. Nat. Commun. 14, 718 (2023).
Guan, Z. Y. et al. Mechanistic insights into the regulation of plant phosphate homeostasis by the rice SPX2-PHR2 complex. Nat. Commun. 13, 1581 (2022).
Zhou, J. et al. Mechanism of phosphate sensing and signaling revealed by rice SPX1-PHR2 complex structure. Nat. Commun. 12, 7040 (2021).
Wege, S. et al. The EXS ___domain of PHO1 participates in the response of shoots to phosphate deficiency via a root-to-shoot signal. Plant Physiol. 170, 385–400 (2016).
Wang, Y., Ribot, C., Rezzonico, E. & Poirier, Y. Structure and expression profile of the Arabidopsis PHO1 gene family indicates a broad role in inorganic phosphate homeostasis. Plant Physiol. 135, 400–411 (2004).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Holm, L. & Sander, C. Protein-structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993).
Chovancova, E. et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. Plos Comput. Biol. 8, e1002708 (2012).
Sun, L. F. et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490, 361–366 (2012).
Popova, Y., Thayumanavan, P., Lonati, E., Agrochao, M. & Thevelein, J. M. Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc. Natl. Acad. Sci. USA 107, 2890–2895 (2010).
Lopez-Sanchez, U. et al. Characterization of XPR1/SLC53A1 variants located outside of the SPX ___domain in patients with primary familial brain calcification. Sci. Rep. 9, 6776 (2019).
Guo, X. X. et al. Spectrum of SLC20A2, PDGFRB, PDGFB, and XPR1 mutations in a large cohort of patients with primary familial brain calcification. Hum. Mutat. 40, 392–403 (2019).
Orimo, K. et al. A Japanese family with idiopathic basal ganglia calcification carrying a novel XPR1 variant. J. Neurol. Sci. 451, 120732 (2023).
Hurlimann, B. C., Stadler-Waibel, M., Werner, T. P. & Freimoser, F. M. Pho91 is a vacuolar phosphate phosphate and polyphosphate Saccharomyces cerevisiae. Mol. Biol. Cell 18, 4438–4445 (2007).
Poirier, Y., Thoma, S., Somerville, C. & Schiefelbein, J. A. Mutant of arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 97, 1087–1093 (1991).
Stefanovic, A. et al. Over-expression of PHO1 in Arabidopsis leaves reveals its role in mediating phosphate efflux. Plant J. 66, 689–699 (2011).
Xu, C. et al. A phosphate-sensing organelle regulates phosphate and tissue homeostasis. Nature 617, 798–806 (2023).
Quistgaard, E. M., Low, C., Guettou, F. & Nordlund, P. Understanding transport by the major facilitator superfamily (MFS): structures pave the way. Nat. Rev. Mol. Cell Biol. 17, 123–132 (2016).
Drew, D., North, R. A., Nagarathinam, K. & Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 121, 5289–5335 (2021).
Shears, S. B. Inositol pyrophosphates: why so many phosphates? Adv. Biol. Regul. 57, 203–216 (2015).
Nagpal, L., He, S., Rao, F. & Snyder, S. H. Inositol pyrophosphates as versatile metabolic messengers. Annu. Rev. Biochem. 93, 317–338 (2024).
Yan, R. et al. Human XPR1 structures reveal phosphate export mechanism. Nature 633, 960–967 (2024).
Lu, Y. et al. Structural basis for inositol pyrophosphate gating of the phosphate channel XPR1. Science 386, eadp3252 (2024).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zhong, E. D., Bepler, T., Berger, B. & Davis, J. H. CryoDRGN: reconstruction of heterogeneous cryo-EM structures using neural networks. Nat. Methods 18, 176–185 (2021).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EMEM density maps. Nat. Methods 11, 63–65 (2014).
Brown, A. et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. Sect. D. Struct. Biol. 71, 136–153 (2015).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. Sect. D. Struct. Biol. 74, 531–544 (2018).
Williams, C. J. et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Jo, S., Kim, T., Iyer, V. G. & Im, W. Software news and updates—CHARNIM-GUI: a web-based grraphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Olsson, M. H. M., Sondergaard, C. R., Rostkowski, M. & Jensen, J. H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 7, 525–537 (2011).
Páll, S., Abraham, M. J., Kutzner, C., Hess, B. & Lindahl, E. Tackling exascale software challenges in molecular dynamics simulations with GROMACS. 2nd International Conference on Exascale Applications and Software (EASC) 3–27 (2015).
Bonomi, M. et al. Promoting transparency and reproducibility in enhanced molecular simulations. Nat. Methods 16, 670–673 (2019).
Briones, R., Biau, C., Kutzner, C., de Groot, B. L. & Aponte-Santamaría, C. GROmaρs: a GROMACS-based toolset to analyze density maps derived from molecular dynamics simulations. Biophys. J. 116, 4–11 (2019).
Acknowledgements
We thank the Cryo-EM Center, the University of Science and Technology of China (USTC), for the EM facility support. We are grateful to Dr. Yongxiang Gao (USTC) for technical support during EM image acquisition. We thank the Center for Protein Research, and Dr. Jianbo Cao at the Public Laboratory of Electron Microscopy, Huazhong Agricultural University, for technical support. We thank the Information Technology Center and State Key Lab of CAD&CG, Zhejiang University, for computational support. We thank Prof. Michael Hothorn and Prof. Xuewen Cheng for helpful suggestions on the manuscript. This work was supported by the National Key Research and Development Program of China (2023YFF1000702 to L.M.), the National Natural Science Foundation of China (32071226 to Z.L., 32422041 to Z.L. and 32371300 to Y.W.), the Foundation of Hubei Hongshan Laboratory (2021HSZD011 to P.Y.), the Fundamental Research Funds for the Central Universities (2662023PY001 to P.Y. and Z.L.), the HZAU-AGIS Cooperation Fund (SZYJY2022022 to Z.L.), and the Zhejiang Provincial National Science Foundation of China (No. LZ24C050003 to Y.W.). Z.G. acknowledges the support of National Postdoctoral Program for Innovative Talents (BX2021108).
Author information
Authors and Affiliations
Contributions
Z.L. conceived and supervised the project. W.Z., Y.C., and Z.G. designed all experiments. W.Z. prepared samples. Y.C. performed transport assays. Z.G. determined the structures. Y.W. performed the MD simulations. M.T., Z.D., J.Z., M.C., J.Z., and Y.L. contributed to plasmids constructing and data collecting. Q.W., Y.L., D.Z., P.Y., and L.M. contributed in data analysis. Z.L. and Y.C. wrote the manuscript with help from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kenji Inaba and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, W., Chen, Y., Guan, Z. et al. Structural insights into the mechanism of phosphate recognition and transport by XPR1. Nat Commun 16, 18 (2025). https://doi.org/10.1038/s41467-024-55471-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55471-9
This article is cited by
-
Transport and InsP8 gating mechanisms of the human inorganic phosphate exporter XPR1
Nature Communications (2025)
-
Structure and function of human XPR1 in phosphate export
Nature Communications (2025)