Abstract
We conducted a multicenter, partially randomized, platform trial to assess the effectiveness of a booster dose of an aerosolized or intramuscular adenovirus type 5 vectored COVID-19 vaccine (Ad5-nCoV) in Chinese adults (NCT05855408). Between May 23, 2023, and August 28, 2023, 4089 eligible participants were equally randomized to receive either a booster dose of aerosolized Ad5-nCoV via oral inhalation at 0.1 mL (IH Ad5-nCoV, n = 2039) or an intramuscular injection of Ad5-nCoV at 0.5 mL (IM Ad5-nCoV, n = 2050). Additionally, 2008 participants who declined the booster but consented to participate in COVID-19 surveillance were enrolled in the control group. All participants were monitored for symptomatic COVID-19 over a six-month surveillance period for the primary outcome. From 14 days after the vaccination, 14 (15/1000 person-years), 19 (20/1000 person-years), and 34 (37/1000 person-years) COVID-19 cases were confirmed in the IH Ad5-nCoV group, the IM Ad5-nCoV group, and the control group, respectively, which resulted in an adjusted effectiveness of 52.3% (95% CI 10.4 to 74.6) for IH Ad5-nCoV and 37.2% (95% CI -11.2 to 64.5) for IM Ad5-nCoV. The IH Ad5-nCoV booster was associated with a lower incidence of symptomatic COVID-19, but there is no solid evidence that IH Ad5-nCoV was more effective than IM Ad5-nCoV.
Similar content being viewed by others
Introduction
The current global population exhibits a multifaceted immunological landscape regarding COVID-19, encompassing individuals who have received COVID-19 vaccinations, those who have been previously infected and recovered from the SARS-CoV-2 virus, and potentially those possessing hybrid immunity1. This intricate hybrid immune status arises from variations in vaccine types administered, dosing regimens, exposure to diverse viral variants, and the timing of these events2. Despite the World Health Organization (WHO) recommendation to utilize a monovalent XBB.1 descendent lineage as the vaccine antigen in May 2023, they concurrently advised against delaying vaccination programs that include WHO emergency-use listed or prequalified COVID-19 vaccines in anticipation of accessing vaccines with updated compositions3. Furthermore, the vast scope of global vaccine inequity will continue to have profound repercussions worldwide, particularly among the most vulnerable populations4,5. Consequently, the inquiry into whether booster doses of COVID-19 vaccines containing the ancestral strain can still afford protection to populations with hybrid immunity during the period of omicron variant dominance remains a crucial area meriting further investigation6.
In China, an impressive over 90% of the population has achieved primary immunization against COVID-19, primarily through the administration of inactivated vaccines7. The first national-scale outbreak of the SARS-CoV-2 omicron predominance occurred at the end of the year 2022 in China, shortly after the dismantling of the “dynamic zero-case policy”8. A survey conducted across 31 provinces in China reported that 82.4% of individuals were infected with SARS-CoV-2 between December 2022 and February 20239. Consequently, a vast majority of the Chinese population now boasts hybrid immunity to COVID-19, derived from both COVID-19 vaccination and breakthrough infections caused by the SARS-CoV-2 omicron variants. Nonetheless, despite a considerable segment of the population acquiring a certain degree of immunity towards COVID-19 via vaccination, prior infection, or a blend of both, the mutation and widespread dissemination of the SARS-CoV-2 virus persist worldwide, giving rise to the advent of novel variants10.
Estimating the effectiveness of COVID-19 booster vaccinations in a predominantly hybrid-immune population is imperative for steering future vaccination strategies aimed at mitigating the spread of COVID-19. In this context, we report on the effectiveness of boost with an aerosolized or intramuscular adenovirus type 5 vectored COVID-19 vaccine (Ad5-nCoV), which has been granted emergency use authorization, in a population predominantly exhibiting hybrid immunity in China.
Results
Trial profile
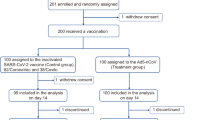
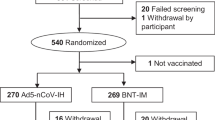
Between May 23, 2023, and August 28, 2023, a total of 4089 eligible participants were equally randomized to receive a booster dose of aerosolized Ad5-nCoV via oral inhalation at 0.1 mL (IH Ad5-nCoV, n = 2039) or intramuscular injection of Ad5-nCoV at 0.5 mL (IM Ad5-nCoV, n = 2050). Additionally, 2008 participants who declined the booster vaccination but consented to participate in COVID-19 surveillance were enrolled as the control group. The final database was locked on March 05, 2024, after a 6-month follow-up period for participants in both the boosting groups and the control group. A total of 6097 participants were included in the final analysis (Fig. 1).
The median follow-up time for both vaccine groups and the control group was 168.0 days (IQR 168.0–180.0). The mean age of all participants was 53.1 years (SD 17.5; range 18–96), with 2902 (47.6%) individuals aged 18–54 years, and 3195 (52.4%) aged 55 years or older (Table 1). The demographic information for the two age groups is detailed in Table S1. Among the 6097 participants, 2821 (46.3%) were male, 1569 (25.7%) reported coexisting conditions and 4555 (74.7%) reported previous SARS-CoV-2 infection history at enrollment. Baseline characteristics were largely similar between the IH Ad5-nCoV and IM Ad5-nCoV groups, but differences were observed between both vaccine groups and the control group (p < 0.05). Specifically, participants in the control group were younger, with a higher proportion of female and SARS-CoV-2 infection history, a higher body mass index (BMI), and a lower proportion of coexisting conditions. In the immunogenicity subgroup, demographic characteristics were well-balanced between the IH Ad5-nCoV and IM Ad5-nCoV groups (Table S2).
Effectiveness analysis
During the surveillance period, a total of 79 COVID-19 cases were confirmed, with 22 (23/1000 person-years) in the IH Ad5-nCoV group, 23 (24/1000 person-years) in the IM Ad5-nCoV group, and 34 (37/1000 person-years) in the control group. However, 5 COVID-19 cases (4 in the IH Ad5-nCoV group and 1 in the IM Ad5-nCoV group) were confirmed within 7 days post-vaccination and excluded from the analysis of the primary and secondary endpoints. As shown in Fig. 2, starting from 28 days post-vaccination, the cumulative COVID-19 incidence of both vaccine groups gradually showed differences compared to the control group.
In the full analysis population, from 14 days after the vaccination, 14 (15/1000 person-years) COVID-19 cases were confirmed in the IH Ad5-nCoV group, 19 (20/1000 person-years) cases were confirmed in the IM Ad5-nCoV group, and 34 (37/1000 person-years) cases were confirmed in the control group, which resulted in adjusted effectiveness of 52.3% (95% CI 10.4–74.6) for IH Ad5-nCoV and 37.2% for IM Ad5-nCoV (95% CI −11.2 to 64.5; Table 2). From 7 days after the vaccination, the adjusted effectiveness of IH Ad5-nCoV and IM Ad5-nCoV was 39.9% (95% CI −7.4 to 66.3) and 28.9% (95% CI −22.8 to 58.8), respectively. All COVID-19 cases confirmed during the surveillance period were symptomatic but mild, thus rendering an assessment of vaccine effectiveness against severe COVID-19 infeasible. Among the 18 successfully sequenced nucleic acid samples derived from COVID-19 endpoint cases, all were typed as Omicron variants, of which 9 (50.0%) were HK.3, 4 (22.2%) were EG.5.1.1, 3 (16.7%) were FY.3.1, 1 (5.6%) was FY.3, and 1 (5.6%) was GF.1.
The adjusted effectiveness of both vaccines in participants aged ≥55 years was numerically lower than that observed in those aged 18–54 years from 14 days after the vaccination, which was 34.5% [95% CI −116.3 to 80.2] vs. 60.2% [95% CI 14.8–81.4] in IH Ad5-nCoV group, and 10.8% [95% CI −166.9 to 70.2] vs. 45.9% [95% CI −7.5 to 72.8] in IM Ad5-nCoV group. Besides, numerically lower adjusted effectiveness of both vaccine groups was observed in participants with coexisting conditions compared to those without (IH Ad5-nCoV: 47.5% [95% CI −123.3 to 87.7] vs. 54.7% [95% CI 8.4–77.6]; IM Ad5-nCoV: 33.7 [95% CI −150.0 to 82.4] vs. 41.0% [95% CI −11.6 to 68.8]). Similarly, numerically lower adjusted effectiveness was found in those without SARS-CoV-2 infection history compared to those with (IH Ad5-nCoV: 50.6% [95% CI −199.6 to 91.9] vs. 52.4% [95% CI 6.8–75.6]; IM Ad5-nCoV: 5.0% [95% CI −328.2 to 78.9] vs. 40.3% [95% CI −11.2 to 67.9]; Table 2, Fig. S2).
Immunogenicity analysis
All participants in the immunogenicity subgroup (n = 121) donated blood samples at days 0 and 14, 118 (97.5%) and 115 (95.0%) of them donated blood samples at months 3 and 6, respectively. At baseline, 65.0% of participants in the IM Ad5-nCoV group and 75.4% of participants in the IH Ad5-nCoV group exhibited an inhibition of ≥50% against wild-type SARS-CoV-2. Furthermore, 40.0–45.0% of participants in the IM Ad5-nCoV group and 50.8–59.0% of participants in the IH Ad5-nCoV group demonstrated inhibition of ≥50% against BA.4, BA.5, and BF.7 variants. However, only 25.0% of participants in the IM Ad5-nCoV group and 36.1% of participants in the IH Ad5-nCoV group showed inhibition of ≥50% against the XBB.1 variant (Fig. 3). 14 days after the booster dose, both vaccines elicited even higher ACE2-RBD binding inhibition against wild-type SARS-CoV-2. Specifically, 95.0% of participants in the IM Ad5-nCoV group and 88.5% of participants in the IH Ad5-nCoV group achieved an inhibition of ≥50%. 6 months post- booster, the proportion of ACE2-RBD binding inhibition ≥50% in the IM Ad5-nCoV group declined significantly to 64.9%, whereas it remained high at 86.2% in the IH Ad5-nCoV group. Regarding the XBB.1 variant, the proportion of ACE2-RBD binding inhibition ≥50% peaked at 14 days in the IM Ad5-nCoV group, with 63.3% of participants achieving this level. In contrast, for the IH Ad5-nCoV group, the peak of ACE2-RBD binding inhibition ≥50% was observed at 3 months post-booster, with 70.0% of participants meeting the criterion. Furthermore, the stratified results based on SARS-CoV-2 infection history indicated that compared to participants with a history of infection, those without SARS-CoV-2 infection history had numerically lower baseline ACE2-RBD binding inhibition against wild-type SARS-CoV-2, BA.5 and XBB.1 variants but exhibited a greater increase 14 days after booster in both vaccine groups (Fig. S5).
ACE2-RBD binding inhibition (%) against spikes of wild-type SARS-CoV-2 (a), BA.1 variant (b), XBB.1 variant (c), BA.2.75 variant (d), BA.2.75.2 variant (e), BA.5 variant (f), BA.4.6 variant (g), BF.7 variant (h), BQ.1 variant (i), and BQ.1.1 variant (j). Lines indicate the median and error bars are interquartile ranges. The horizontal dashed line is the 50% inhibition. IM Ad5-nCoV: day 0 (n = 60), day 14 (n = 60), month 3 (n = 58), month 6 (n = 57); IH Ad5-nCoV: day 0 (n = 61), day 14 (n = 61), month 3 (n = 60), month 6 (n = 58). No technical replicates. The p values are the results of comparison between different time points using two-sided generalized estimating equations (day 0 versus day 14 and month 3 versus month 6). The p values for multiple comparisons have been adjusted. IM Ad5-nCoV adenovirus type 5 vectored COVID-19 vaccine through intramuscular injection, IH Ad5-nCoV adenovirus type 5 vectored COVID-19 vaccine through oral inhalation.
Pseudovirus neutralization results revealed that both vaccine groups exhibited relatively high geometric mean titers (GMTs) of antibody at baseline against wild-type SARS-CoV-2 (IH Ad5-nCoV: 550.4; IM Ad5-nCoV: 411.0) and BA.4/5 (IH Ad5-nCoV: 451.9; IM Ad5-nCoV: 300.9). After vaccination, pseudovirus neutralization antibody titers against wild-type SARS-CoV-2 in the IH Ad5-nCoV group moderately increased from 14 days and peaked at 3 months after vaccination, then slightly declined at 6 months, with GMTs of 796.9 (95% CI 635.6–999.1), 1026.2 (95% CI 792.7–1328.6) and 880.9 (95% CI 700.9–1107.2), respectively (Fig. 4). Compared with the GMTs of pseudovirus neutralization antibodies against wild-type SARS-CoV-2 in the IH Ad5-nCoV group, those in the IM Ad5-nCoV group were comparable at 14 days, but numerically lower at 3 and 6 months, with GMTs of 796.4 (95% CI 635.3–998.2), 681.6 (95% CI 542.2–856.9) and 520.0 (95% CI 413.1–654.6), respectively. In contrast to pseudovirus neutralization antibodies against wild-type SARS-CoV-2, those against BA.4/5 achieved comparable levels, with the peak GMTs at 3 months of 1061.0 (95% CI 800.1–1405.5) in the IH Ad5-nCoV group and 883.0 (95% CI 670.1–1163.4) in the IM Ad5-nCoV group. Although pseudovirus neutralization antibodies against XBB.1.16 were relatively lower in both vaccine groups compared to those against wild-type and BA.4/5 SARS-CoV-2, GMTs increased significantly after booster immunization, from 79.3 at baseline to the peak of 317.0 in the IH Ad5-nCoV group, and from 65.3 at baseline to the peak of 302.8 in the IM Ad5-nCoV group, both at 3 months. Compared to participants with a history of infection, those without SARS-CoV-2 infection history had numerically lower pseudovirus neutralization antibodies against wild-type SARS-CoV-2 and BA.4/5 variant but exhibited a greater increase 14 days after booster in both vaccine groups (Fig. S6). However, no difference in pseudovirus neutralization antibodies was observed against XBB.1.16 between participants with and without SARS-CoV-2 infection history.
Pseudovirus neutralizing antibody titers against wild-type SARS-CoV-2 (a), BA.4/5 variant (c), and XBB.1.16 (e) in the IM Ad5-nCoV group. Pseudovirus neutralizing antibody titers against wild-type SARS-CoV-2 (b), BA.4/5 variant (d), and XBB.1.16 (f) in the IH Ad5-nCoV group. IM Ad5-nCoV: day 0 (n = 60), day 14 (n = 60), month 3 (n = 58), month 6 (n = 57); IH Ad5-nCoV: day 0 (n = 61), day 14 (n = 61), month 3 (n = 60), month 6 (n = 58). No technical replicates. The p values are the results of comparison between different time points using two-sided mixed-effects models (day 0 versus day 14 and month 3 versus month 6). The p values for multiple comparisons have been adjusted. IM Ad5-nCoV adenovirus type 5 vectored COVID-19 vaccine through intramuscular injection, IH Ad5-nCoV adenovirus type 5 vectored COVID-19 vaccine through oral inhalation, GMT geometric mean antibody titer.
Enzyme-linked immunospot (ELISpot) responses against wild-type SARS-CoV-2 were detectable before the booster dose in over 90% of participants within both vaccine groups, with a mean number of spot-forming cells per 2 × 105 cells of 25.8 (95% CI 19.6–31.9) for IFN-γ and 10.2 (95% CI 7.2–13.1) for IL-2 in the IM Ad5-nCoV group, 27.8 (23.0–32.6) for IFN-γ and 6.7 (95% CI 5.1–8.6) for IL-2 in the IH Ad5-nCoV group (Fig. 5). After the booster vaccination, the levels of IFN-γ and IL-2 against wild-type SARS-CoV-2 remained comparable to baseline levels at 14 days and 3 months but showed a slight decline at 6 months. In the IH Ad5-nCoV group, the mean number of spot-forming cells per 2 × 105 cells for IFN-γ was 22.3 (95% CI 13.5–31.0) at 14 days, 22.8 (95% CI 12.6–32.9) at 3 months, and 10.6 (95% CI 5.4–15.8) at 6 months, and that for IL-2 was 9.1 (95% CI 5.9–12.2) at 14 days, 7.9 (95% CI 5.8–9.9) at 3 months, and 3.7 (95% CI 0.6–6.6) at 6 months. In the IM Ad5-nCoV group, the mean number of spot-forming cells per 2 × 105 cells for IFN-γ was found to be 27.3 (95% CI 15.4–39.1) at 14 days, 23.9 (95% CI 17.8–29.9) at 3 months, and 14.9 (95% CI 5.0–24.9) at 6 months, and that for IL-2 was 10.5 (95% CI 7.7–13.4) at 14 days, 8.4 (95% CI 5.9–10.8) at 3 months, and 4.3 (95% CI 1.9–6.8) at 6 months. ELISpot responses against BA.4/5 at baseline were similar to those against wild-type SARS-CoV-2 and were detectable in over 90% of participants in both vaccine groups. After the booster vaccination, no elevation in the levels of IFN-γ and IL-2 was observed as well. Besides, no difference in ELISpot responses was observed among participants with versus without SARS-CoV-2 infection history (Fig. S7).
ELISpot responses for IFN-γ against wild-type SARS-CoV-2 (a) and BA.4/5 variant (b). ELISpot responses for IL-2 against wild-type SARS-CoV-2 (c) and BA.4/5 variant (d). Lines indicate the mean and error bars are 95% CIs. IM Ad5-nCoV against wild-type SARS-CoV-2: day 0 (n = 60), day 14 (n = 60), month 3 (n = 56), month 6 (n = 31); IH Ad5-nCoV against wild-type SARS-CoV-2: day 0 (n = 61), day 14 (n = 61), month 3 (n = 60), month 6 (n = 34). IM Ad5-nCoV against BA.4/5 variant: day 0 (n = 47), day 14 (n = 39), month 3 (n = 58), month 6 (n = 57); IH Ad5-nCoV against BA.4/5 variant: day 0 (n = 54), day 14 (n = 43), month 3 (n = 26), month 6 (n = 59). No technical replicates. The p values are the results of comparison between different time points using two-sided mixed-effects models (day 0 versus day 14 and month 3 versus month 6). The p values for multiple comparisons have been adjusted. IM Ad5-nCoV adenovirus type 5 vectored COVID-19 vaccine through intramuscular injection. IH Ad5-nCoV adenovirus type 5 vectored COVID-19 vaccine through oral inhalation, IFN interferon, IL interleukin, SFCs spot forming cells, PBMCs peripheral blood mononuclear cells.
The pattern of RBD-specific IgG antibody results was consistent with that of pseudovirus neutralization antibody. Specifically, the geometric mean concentration (GMC) of RBD-specific IgG antibody against wild-type SARS-CoV-2 exhibited a notable increase after vaccination in both vaccine groups, from 1071.2 (95% CI 833.1–1377.3) BAU/mL at baseline to a peak of 2174.1 (95% CI 1890.4–2500.5) BAU/mL at 14 days in the IM Ad5-nCoV group, from 1635.9 (95% CI 1339.0–1998.7) BAU/mL at baseline to a peak of 2779.8 (95% CI 2432.1–3177.2) BAU/mL at 3 months in the IH Ad5-nCoV group. In comparison, RBD-specific IgG antibody levels against BA.4/5 were notably lower, but significant increases were still observed from 14 days post-vaccination compared to baseline in both boosting groups (Fig. S3). Compared to participants with a history of infection, those without SARS-CoV-2 infection history had numerically lower baseline RBD-specific IgG antibody levels against wild-type SARS-CoV-2 and BA.4/5 variant but reached a similar or greater peak level 14 days after booster in both vaccine groups (Fig. S8).
After the booster vaccination, the geometric mean concentration (GMC) of RBD-specific IgA antibody against XBB.1.5 increased significantly in both vaccine groups, from 168.7 U/mL at baseline to the peak of 427.6 U/mL in the IM Ad5-nCoV group, and from 229.1 at baseline to the peak of 477.4 in the IH Ad5-nCoV group, at 14 days (Fig. S4). Although the GMC of RBD-specific IgA antibody against XBB.1.5 decreased slightly, it remained at a relatively high level in both vaccine groups 3 and 6 months after the booster vaccination. Similarly, the stratified results showed that participants without a SARS-CoV-2 infection history had numerically lower baseline RBD-specific IgA antibodies and reached similar peak levels 14 days after the booster compared with those with a history of infection (Fig. S9).
Safety analysis
During the six-month follow-up period after the booster vaccination, 25 episodes of serious adverse events (SAEs) were reported in 22 participants, within 9 (0.4%) of 2050 participants from the IM Ad5-nCoV group, 7 (0.3%) of 2039 participants from the IH Ad5-nCoV group, and 6 (0.3%) of 2008 participants from the control group (Table S3). The incidence of SAEs did not significantly differ between the two vaccine groups and the control group (IM Ad5-nCoV vs. control: p = 0.4618; IH Ad5-nCoV vs. control: p = 0.8025). Among the 19 SAEs reported in both vaccine groups, the most common were respiratory thoracic and mediastinal disorders (6, 31.6%) and nervous system disorders (4, 21.1%). The one with an allergic reaction in the IH Ad5-nCoV group was considered related to the vaccination, while the remaining SAEs were not vaccine-related. A comprehensive list of all SAEs is provided in Table S4.
Discussion
In this trial, we found that boosting with the IH Ad5-nCoV containing the ancestral strain was associated with a lower incidence of symptomatic COVID-19 caused by Omicron variants, despite the majority of the individuals having a hybrid-immune background against COVID-19, characterized by both detectable humoral and cellular immunity at enrollment. Specifically, the adjusted effectiveness of the IH Ad5-nCoV was found to be 52.3% (95% CI 10.4–74.6), whereas the IM Ad5-nCoV showed a lower effectiveness of 37.2% (95% CI −11.2 to 64.5). Aligning with our findings, previous studies have also demonstrated that a booster dose of an mRNA COVID-19 vaccine containing the ancestral strain can offer protection against the Omicron variant. A retrospective cohort study conducted in the United States revealed that a single booster dose of any mRNA COVID-19 vaccine reduced the risk of COVID-19 caused by the Omicron variant among individuals who had been prime with mRNA COVID-19 vaccines but had not previously been infected (hazard ratio [HR], 0.43; 95% CI, 0.41–0.46) as well as those who had been previously infected (HR, 0.66; 95% CI, 0.58–0.76)11. Similarly, another prospective cohort study indicated that among previously uninfected participants who received the BNT162b2 vaccine, the adjusted vaccine effectiveness approximately 6 months post-booster was 51% (95% CI, 22–69)12.
Furthermore, our findings suggest that the inhaled version of Ad5-nCoV may confer a numerically greater protection against COVID-19 compared to the intramuscular injected version of Ad5-nCoV (52.3% vs. 37.2%) but not significantly, despite similar cellular and humoral responses were observed across both vaccine groups and 4/5 lower of the dosage of the IH Ad5-nCoV compared to that of the IM Ad5-nCoV. This is the first study demonstrating a head-to-head comparison of vaccine effectiveness between two immunization routes of Ad5-nCoV. Our results imply that individuals in the general population who possess hybrid immunity due to both primary vaccination and SARS-CoV-2 infection may benefit more from enhanced synergy between systemic and mucosal immunity via the administration of additional mucosal vaccines. Previous studies have indicated that the IH Ad5-nCoV induces more robust mucosal IgA responses compared to the IM Ad5-nCoV in recipients of inactivated COVID-19 vaccines13,14. In addition, an increasing pattern in mucosal immunity resident memory T-cells was also observed after orally administered aerosolized Ad5-nCoV15. These observations could potentially explain the higher effectiveness of IH Ad5-nCoV in populations predominantly with established hybrid immunity against COVID-19.
Moreover, we observed a numerically decreased effectiveness of both vaccines in participants aged ≥55 years, as well as those with coexisting conditions. Despite this, the adjusted effectiveness for the IH Ad5-nCoV in those aged ≥55 years and with coexisting conditions remained at 34.5% (95% CI −116.3 to 80.2) and 47.5% (95% CI −123.3 to 87.7). This finding supports the necessity for a booster dose among individuals at heightened risk of infection with omicron variants and aligns with the updated recommendations on COVID-19 vaccination issued by the World Health Organization’s Strategic Advisory Group on Immunization16. Notably, a numerically lower effectiveness was observed in both vaccine groups among participants without a SARS-CoV-2 infection history compared to those with. This observation corresponds with existing literature, which suggests that hybrid immunity, resulting from both infection and vaccination, engenders stronger and more broad-spectrum immune responses, with high-quality memory B cells generated at 5–10 times higher than those achieved through infection or vaccination alone, and protection against symptomatic disease lasting for 6–8 months17,18. However, it is important to note that caution should be exercised in interpreting this finding, given the relatively small proportion of participants in the study who lacked a history of SARS-CoV-2 infection.
The results from ACE2-RBD-binding inhibition, pseudovirus neutralization assays, and RBD-specific IgG antibody tests indicated that a significant majority of participants in both vaccine groups exhibited immune responses not only to wild-type SARS-CoV-2 but also to BA.4/5 and BF.7 variants at baseline. This is consistent with the background that a large portion of the Chinese population had received COVID-19 vaccines targeting the wild-type strain, and that Omicron BA.5.2 and BF.7 were the dominant strains circulating in China at the end of 202219. After the booster vaccination, the ACE2-RBD binding inhibition and pseudovirus neutralization levels in the IM Ad5-nCoV group rapidly reached their peak primarily 14 days after boosting. In contrast, the IH Ad5-nCoV group showed a peak at 3 months with a relatively slower rising speed in our study, which concurs with the results reported in a previous phase 4 trial evaluating the immunogenicity of a second booster of either IH Ad5-nCoV or IM Ad5-nCoV following three doses of CoronaVac in China14. Notably, although the booster vaccines used in our study were based on the ancestral strain of SARS-CoV-2, favorable cross-neutralizing against several Omicron subvariants, including B.1.1.529, BA.2.75, BA.2.75.2, BA.5, BA.4.6, BF.7, BQ.1, BQ.1.1 and XBB.1, was observed following booster vaccination.
Despite the significant addition of protection and humoral responses elicited by the booster dose of IH Ad5-nCoV or IM Ad5-nCoV, our study did not observe any significant T-cell responses after the boosting. One plausible explanation for this observation is that the already baseline levels of T-cell responses may impede further augmentation of cellular responses. According to our previously reported phase 4 trial of IH Ad5-nCoV or IM Ad5-nCoV given as the second booster following three doses of inactivated COVID-19 vaccines in adults with no SARS-CoV-2 exposure history, we found that participants in the IH Ad5-nCoV group had median IFN-γ+ spot counts of 117 (IQR 3–437) and IL-2+ spot counts of 83 (20–393) per 106 peripheral blood mononuclear cells (PBMCs), whereas, the IM Ad5-nCoV showed an even lower response14. These values are similar to the baseline IFN-γ and IL2 ELISpot levels observed in this platform trial. Furthermore, in a previously reported study, cellular immunity in COVID-19 patients who convalesced from SARS-CoV-2 infection persisted with no significant decrease for at least seven months post-infection20.
In May 2023, the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) recommended the utilization of a monovalent vaccine antigen derived from the XBB.1 descendent lineage, specifically XBB.1.53. Following this recommendation, several COVID-19 vaccines formulated with the monovalent XBB.1.5 antigen have been approved for use by regulatory authorities and introduced into COVID-19 vaccination programs in several countries, including mRNA, protein-based, and viral vector vaccines21,22. As of April 2024, the TAG-CO-VAC has recommended the adoption of a monovalent JN.1 lineage as the antigen for future COVID-19 vaccine formulations23. Nevertheless, in accordance with the WHO Strategic Advisory Group of Experts on Immunization policy, vaccination programs should continue utilizing any COVID-19 vaccines that are listed for emergency use or prequalified by the WHO. It is crucial that vaccination schedules should not be delayed in anticipation of access to vaccines with updated compositions. Furthermore, factors such as vaccine prices and cold chain systems have further hindered the accessibility of COVID-19 vaccines, particularly the newly updated monovalent vaccines containing the XBB.1 variant, in low- and middle-income countries24. Our study provides evidence supporting the use of the IH Ad5-nCoV containing the ancestral strain as an alternative option when the updated COVID-19 vaccine containing a new variant is not available.
However, there are some limitations to our study. First, all COVID-19 endpoint cases in our study were symptom-driven, and thus we were unable to ascertain the protection of a booster dose of IH Ad5-nCoV or IM Ad5-nCoV against asymptomatic SARS-COV-2 infection. Second, the COVID-19 endpoint cases identified in our trial were all mild in severity, precluding an evaluation of the effectiveness of both vaccines against severe disease or hospital admission. Third, participants of the control group were not randomized. Therefore, we calculated the effectiveness adjusted for age, sex, BMI, and SARS-CoV-2 infection history. However, covariate adjustment may not fully balance other unmeasured confounding factors at baseline between the vaccine groups and the control group, and given the wide confidence interval for the estimated effectiveness, the results should be interpreted with caution. Fourth, the lack of data on mucosal IgA responses restricts our ability to fully compare the immunogenicity of IH Ad5-nCoV and IM Ad5-nCoV, which deserves further exploration in future studies. Furthermore, we did not evaluate the immune responses of the participants who experienced breakthrough infection during the surveillance period, which merits further research. Finally, both vaccines evaluated in this study were based on wild-type SARS-CoV-2, and we only observed their effectiveness for a period of 6 months, long-term protection has yet to be established.
In conclusion, our study showed that a boost with the ancestral strain containing vaccine IH Ad5-nCoV was associated with a lower incidence of symptomatic COVID-19 caused by Omicron variants, in adults predominantly with hybrid immunity against SARS-CoV-2. Although IH Ad5-nCoV showed a numerically higher estimated effectiveness than IM Ad5-nCoV did, there is no solid evidence that the inhaled version of aerosolized Ad5-nCoV is more favorable than an intramuscular injection of Ad5-nCoV.
Methods
Study design and participants
This is a multicenter, partially randomized, platform trial that aims to evaluate the effectiveness of a booster dose of aerosolized or intramuscular Ad5-nCoV in adults aged 18 years and older predominantly with hybrid immunity against COVID-19, through a six-month surveillance period. Participants were recruited from five cities, including Taizhou, Changzhou, Lianyungang, Suqian, and Wuxi, in Jiangsu province, China. The study protocol and informed consent form were reviewed and approved by the Ethics Committee of Jiangsu Provincial Center for Disease Control and Prevention. The study was registered with ClinicalTrials.gov under the identifier NCT05855408 and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Eligible participants were individuals aged 18 years and older, including those over 60 years of age and individuals with underlying medical conditions. Eligibility required an interval ≥4 months after the previous SARS-CoV-2 infection or confirmation of never having been infected, as well as ≥6 months since the last COVID-19 vaccination. Previous SARS-CoV-2 infection was reported by the participants themselves and defined as having a positive antigen rapid test or nucleic acid test result or having acute onset of at least two typical symptoms or signs of COVID-19 with a history of exposure to probable or confirmed COVID-19 cases but without an etiological test result. Participants who were willing to receive a booster dose were randomly assigned to receive one of the boost vaccines, while those who declined the booster vaccination but consented to participate in COVID-19 surveillance were included as the control group. Exclusion criteria for both vaccine groups included individuals exhibiting suspected COVID-19 symptoms on the day of enrollment, positive results on antigen rapid tests for SARS-CoV-2, having already completed a second COVID-19 booster immunization, a history of severe adverse reactions or anaphylaxis related to vaccination, and pregnant or lactating women. For the control group, the exclusion criteria were identical to those for the vaccine groups, with the exception of not having a history of severe adverse reactions or anaphylaxis related to vaccination. A complete list of inclusion and exclusion criteria is detailed in the study protocol. All participants in both the vaccine and control groups provided written informed consent prior to screening. Participants from the immunogenicity subgroup were provided with compensation due to the blood collection.
Randomization and masking
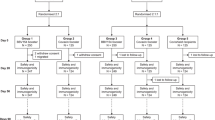
Randomization was executed using an interactive web response system (IWRS), based on a blocked randomization list generated by an independent statistician utilizing SAS software (version 9.4). Eligible participants who consented to receive a COVID-19 booster dose were randomly assigned via IWRS to receive one of the two COVID-19 vaccines, the aerosolized Ad5-nCoV or intramuscular Ad5-nCoV, both manufactured by CanSino Biologics (Convidecia Air™ and Convidecia™). Furthermore, stratified randomization was implemented based on age categories (18-59 years or 60 years and above) and participants’ history of SARS-CoV-2 infection. The design diagram of this study is illustrated in Fig. S1.
Procedures
The IH Ad5-nCoV was orally inhaled at 0.1 mL per dose (1 × 1010 viral particles), using a continuous vaporizing system containing a nebulizer (Aerogen, Galway, Ireland) integrated by Suzhou Weiqi Biological Technology (Suzhou City, China) to aerosolise the Ad5-nCoV and generate the aerosolised droplets of vaccine into a disposable suction cup. In contrast, the IM Ad5-nCoV was administered through an intramuscular injection of 0.5 mL (5 × 1010 viral particles). Both vaccines are the replication-defective Ad5 vectored vaccine expressing the spike glycoprotein of SARS-CoV-2 (Wuhan-Hu-1). Following vaccination, all participants from both vaccine groups were observed at the clinic for a minimum of 30 min to monitor for any immediate adverse reactions.
In this study, all participants were monitored for symptom-driven COVID-19 for 6 months either after the booster dose (in the vaccine groups) or from the time of enrollment (in the control group). This surveillance incorporated both active and passive monitoring strategies. We provided antigen rapid test kits for SARS-CoV-2 infection (Vazyme, China) to both participants in the vaccine and control groups. Participants were instructed to conduct a self-test using the antigen rapid test kits, following the product manual, if they experienced any suspected COVID-19 symptoms during the surveillance period. COVID-19 suspected symptoms include dry throat, sore throat, cough, fever, muscle aches, decreased or loss of smell and taste, nasal congestion, runny nose, diarrhea, conjunctivitis, fatigue, malaise, headache, dyspnea, and nausea. Participants were instructed to perform an antigen rapid test within 24 h after the appearance of the first suspected COVID-19 symptom. If the initial test result was negative, they were required to conduct additional tests at intervals of at least 24 h until achieving three consecutive negative results. In the event of a positive antigen test result, participants were mandated to promptly inform the investigators. Subsequently, the investigators took a throat swab from the positive cases for nucleic acid test within 48 h after receiving the positive rapid test report. Investigators followed up on each positive case through weekly telephone consultations until the resolution of symptoms or recovery. Each COVID-19 episode was classified as mild, moderate, severe, or critical according to the grading standard issued by the National Health Commission of China25. Moreover, investigators conducted weekly telephone follow-ups for a period of 6 months post-vaccination to monitor for serious adverse events and to remind participants to perform self-testing if they experienced any suspected COVID-19 symptoms.
The immunogenicity subgroup consisted of the first 60 participants from each vaccine group. Blood samples were taken for serum and PBMC isolation before the booster dose, subsequently and at 14 days, 3 months, and 6 months post-booster dose. ACE2 inhibition activities were measured using the V-Plex SARS-CoV-2 Panel 32 (ACE2) kits (Meso Scale Discovery, Gaithersburg, MSD, USA [Lot K15671U-2]) at a 1:50 dilution per the manufacturer’s instructions. The spike proteins of wild-type SARS-CoV-2, B.1.1.529, BA.2.75, BA.2.75.2, BA.5, BA.4.6, BF.7, BQ.1, BQ.1.1 and XBB.1 binding to ACE2 were measured both alone and in the presence of sera to calculate percentage inhibition. Neutralizing antibody responses against wild-type SARS-CoV-2, XBB.1.16, and BA.4/5 were assessed using pseudovirus neutralization tests (a human immunodeficiency virus pseudovirus system expressing the spike glycoprotein), with the limit of detection of 1:30. T-cell immune responses were quantified using PBMCs with commercially available Human IFN-γ and IL-2 ELISpot assay kits (BD, Lot 551849 and 551282). PBMCs were stimulated with a pool of peptides spanning the wild-type (Wuhan-Hu-1) and BA.4/5 SARS-CoV-2 spike protein for 20 h at a density of 2×105 cells per well. After stimulation, the plates were incubated with IFN-γ or IL-2-detecting antibodies. Spots representing IFN-γ or IL-2-producing cells were counted using an ImmunoSpot S6 Universal Reader (CTL). The final determinations were calculated by subtracting the negative stimulation background levels from the measured values. For COVID-19 endpoint cases with cycle threshold values below 32, nucleic acid samples underwent sequencing via the next-generation sequencing technology for variant typing of SARS-CoV-2.
In addition to the outcomes specified in the protocol, we also assessed RBD-specific IgG and IgA antibody responses with ELISA kits manufactured by Vazyme Biotech (Nanjing, China). RBD-specific IgG antibody was detected using the quantitative kit, the OD value was determined as the difference between the OD value at 450 nm and OD value at 630 nm, and the lower limit of quantification was 0.125 BAU/mL. The final IgG antibody concentration was calculated by multiplying the detected value with the serum dilution factor. The RBD-specific IgA ELISA kit employs an indirect ELISA method. Following a two-step incubation and color development process, the absorbance measured at a specific wavelength was positively correlated with the level of SARS-CoV-2 RBD IgA antibody against XBB.1.5. Subsequently, the antibody concentration was calculated using a standard curve.
Outcomes
The primary outcome was the incidence of COVID-19 endpoint cases from 14 days to 6 months after receiving the booster dose. COVID-19 endpoint cases were defined as participants with COVID-19 confirmed by positive antigen rapid test or nucleic acid test after receiving the booster dose. The secondary outcomes for effectiveness included the incidence of COVID-19 endpoint cases from 7 days and 28 days to 6 months after receiving the booster dose, as well as the severity of these cases. The secondary outcomes for immunogenicity encompassed ACE2-RBD binding inhibition rates and pseudovirus neutralization antibody levels against wild-type SARS-CoV-2 and Omicron variants at 14 days, 3, and 6 months after the booster vaccination. Specific T-cell responses measured by ELISpot assay were also the secondary outcomes. The secondary outcome for safety was the incidence of serious adverse events within 6 months after the booster administration.
Statistical analysis
We hypothesized that participants in the vaccine group receiving a booster shot would exhibit approximately 50% protection compared to those in the control group who did not receive a booster. The cumulative incidence rate of COVID-19 endpoint cases in the control group over a 6-month period was estimated to be around 5%, while it was projected to be about 2.5% in the vaccine group. Sample size calculation was conducted using group-sequential tests for two proportions using PASS software (version 16.0), applying a one-sided α value of 0.05 and targeting a statistical power of 90%. This analysis indicated that each vaccine group and the control group required a minimum of 1308 participants. Taking into account a potential 30% dropout rate, we set a target enrollment of 2000 participants per group.
Statistical analyses were conducted using SAS version 9.4, with all tests being two-sided at an α value of 0.05. Effectiveness analyses were done in the full analysis population, comprising all randomized participants who either received one dose of the vaccine or were enrolled in the control group). The incidence of COVID-19 in the control group was calculated based on all endpoint cases identified from the day following enrollment. To derive the effectiveness estimates, the Cox proportional hazards regression model was utilized. Moreover, effectiveness adjusted by age, sex, BMI, and SARS-CoV-2 infection history was also calculated. Considering the mean age of all participants, we stratified the effectiveness of the booster vaccination based on age with a cutoff of 55 years. Cumulative incidence data were presented using the Kaplan–Meier method. Immunogenicity analyses were confined to the immunogenicity subgroup, comprising all participants who received vaccinations and provided blood samples after vaccination. Safety analyses were performed on the full analysis population. The χ²-test or Fisher’s exact test was used for categorical demographic data. Student’s t-test was used for continuous demographic data. Mixed-effect models were used for continuous immunogenicity data, and generalized estimating equations were applied for categorical immunogenicity data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The study protocol is available in the Supplementary Information file. Sequencing data have been deposited in the China National Center for Bioinformation under accession number CRA023149 and are accessible at https://bigd.big.ac.cn/gsa/browse/CRA023149. Individual participant data that underlie the results reported in this article are available under restricted access for the requirements imposed by the Chinese Human Genetic Resources Administration concerning the public disclosure of clinical trial data. Researchers who provide a scientifically sound proposal will be allowed access to the de-identified individual participant data. Individual participant data can be obtained with a request to the corresponding authors.
Code availability
No custom code was used.
References
Suryawanshi, R. & Ott, M. SARS-CoV-2 hybrid immunity: silver bullet or silver lining? Nat. Rev. Immunol. 22, 591–592 (2022).
Pušnik, J. et al. SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun. 14, 572 (2023).
World Health Organization. Statement on the antigen composition of COVID-19 vaccines. https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (2023).
Ba, Z. et al. Vaccine inequity-induced COVID-19 dilemma: time to sober up. Leg. Med. (Tokyo) 66, 102364 (2024).
Privor-Dumm, L. et al. Vaccine access, equity and justice: COVID-19 vaccines and vaccination. BMJ Glob. Health 8, https://doi.org/10.1136/bmjgh-2023-011881 (2023).
Pilz, S. & Ioannidis, J. P. A. Does natural and hybrid immunity obviate the need for frequent vaccine boosters against SARS-CoV-2 in the endemic phase? Eur. J. Clin. Investig. 53, e13906 (2023).
Zheng, L., Liu, S. & Lu, F. Impact of National Omicron Outbreak at the end of 2022 on the future outlook of COVID-19 in China. Emerg. Microbes Infect. 12, 2191738 (2023).
Jia, S. et al. Relative effectiveness of a heterologous booster dose with adenovirus type 5 vectored COVID-19 vaccine versus three doses of inactivated COVID-19 vaccine in adults during a nationwide outbreak of omicron predominance, in China: a retrospective, individually matched cohort-control study. Emerg. Microbes Infect. 13, 2332660 (2024).
Fu, D. et al. Effectiveness of COVID-19 vaccination against SARS-CoV-2 omicron variant infection and symptoms—China, December 2022–February 2023. China CDC Wkly 5, 369–373 (2023).
Bates, T. A. et al. An extended interval between vaccination and infection enhances hybrid immunity against SARS-CoV-2 variants. JCI Insight 8, https://doi.org/10.1172/jci.insight.165265 (2023).
Shrestha, N. K. et al. Coronavirus disease 2019 vaccine boosting in previously infected or vaccinated individuals. Clin. Infect. Dis. 75, 2169–2177 (2022).
Hall, V. et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N. Engl. J. Med. 386, 1207–1220 (2022).
Zhang, Z. et al. Boosting with an aerosolized Ad5-nCoV elicited robust immune responses in inactivated COVID-19 vaccines recipients. Front. Immunol. 14, 1239179 (2023).
Tang, R. et al. Safety and immunogenicity of aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or inactivated COVID-19 vaccine CoronaVac given as the second booster following three doses of CoronaVac: a multicentre, open-label, phase 4, randomised trial. Lancet Respir. Med. 11, 613–623 (2023).
Xu, J. W. et al. Safety and immunogenicity of heterologous boosting with orally administered aerosolized bivalent adenovirus type-5 vectored COVID-19 vaccine and B.1.1.529 variant adenovirus type-5 vectored COVID-19 vaccine in adults 18 years and older: a randomized, double blinded, parallel controlled trial. Emerg. Microbes Infect. 13, 2281355 (2024).
World Health Organization. COVID-19 advice for the public: Getting vaccinated. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (2023).
Spinardi, J. R. & Srivastava, A. Hybrid immunity to SARS-CoV-2 from infection and vaccination—evidence synthesis and implications for new COVID-19 vaccines. Biomedicines 11, https://doi.org/10.3390/biomedicines11020370 (2023).
Bobrovitz, N. et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect. Dis. 23, 556–567 (2023).
Zhu, W. et al. Genomic evolution of BA.5.2 and BF.7.14 derived lineages causing SARS-CoV-2 outbreak at the end of 2022 in China. Front. Public Health 11, 1273745 (2023).
Chen, J. et al. Decline in neutralising antibody responses, but sustained T-cell immunity, in COVID-19 patients at 7 months post-infection. Clin. Transl. Immunol. 10, e1319 (2021).
European Commission. Union Register of medicinal products for human use. https://ec.europa.eu/health/documents/community-register/html/h1528.htm (2024).
U.S. Food and Drug Administration. FDA Authorizes Updated Novavax COVID-19 Vaccine Formulated to Better Protect Against Currently Circulating Variants. https://www.fda.gov/news-events/press-announcements/fda-authorizes-updated-novavax-covid-19-vaccine-formulated-better-protect-against-currently (2023).
World Health Organization. Statement on the antigen composition of COVID-19 vaccines. https://www.who.int/news/item/26-04-2024-statement-on-the-antigen-composition-of-covid-19-vaccines (2024).
Ulrichs, T. et al. Changing epidemiology of COVID-19: potential future impact on vaccines and vaccination strategies. Expert Rev. Vaccines 23, 510–522 (2024).
National Health Commission of the People’s Republic of China. Notice on the Diagnosis and Treatment Protocol for SARS-CoV-2 Infection (Trial Version 10). http://www.nhc.gov.cn/xcs/zhengcwj/202301/32de5b2ff9bf4eaa88e75bdf7223a65a.shtml (2023).
Acknowledgements
This work is supported by the National Key Research and Development Program of China (grant number 2023YFC2307601 [J.-X.L.]), National Natural Science Foundation of China (grant numbers 82341031 [F.-C.Z.], 82173584 [J.-X.L.] and 82222062 [J.-X.L.]), Jiangsu Provincial Science Fund for Distinguished Young Scholars (grant number BK20220064 [J.-X.L.]), Jiangsu Provincial Key Project of Science and Technology Plan (grant numbers BE2023601 [F.-C.Z.]), and Fund of State Key Laboratory of Drug Regulatory Science (grant number 2023SKLDRS0114 [Q.-Y.M.] and 2024SKLDRS0205 [Q.H.]). We thank CanSino Biologics for providing the investigational vaccines for this study.
Author information
Authors and Affiliations
Contributions
S.-Y.J., Y.-B.L., and Q.H. were co-first authors. J.-X.L., Q.-Y.M., and F.-C.Z. were joint corresponding authors. J.-X.L. is the principal investigator of this trial. J.-X.L., F.-C.Z., and Y.-B.L. designed the trial and the study protocol. S.-Y.J. drafted the manuscript. Q.H., Z.-L.L., X.-X.Z., and Q.-Y.M. were responsible for laboratory analyses. H.-X.P., L.Z., F.-C.Z., and J.-X.L. contributed to the critical review and revision of the article. H.-X.P. and L.Z. contributed to the data interpretation. J.Z., Y.-Z.P., S.L., J.-J.W., and K.Y. led and participated in the site work, including recruitment, follow-up, and data collection. Y.Z. and M.-Y.L. were responsible for the statistical analysis. S.-M.L., L.C., and A.-H.Y. contributed to the literature search.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bo Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, S., Liu, Y., He, Q. et al. Effectiveness of a booster dose of aerosolized or intramuscular adenovirus type 5 vectored COVID-19 vaccine in adults: a multicenter, partially randomized, platform trial in China. Nat Commun 16, 2969 (2025). https://doi.org/10.1038/s41467-025-58327-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58327-y