Abstract
There is a need for novel therapies for patients with previously treated HER2-positive gastroesophageal adenocarcinoma (GEA). This phase 1 (NCT02892123) dose-escalation and expansion trial evaluated zanidatamab (a dual HER2-targeted bispecific antibody) ± chemotherapy in previously treated patients with HER2-expressing, locally advanced/metastatic cancers. Here, we report the outcomes for GEA cohorts receiving zanidatamab monotherapy or with chemotherapy (paclitaxel or capecitabine). The primary endpoint was safety and tolerability. Secondary endpoints were objective response rate (ORR), disease control rate, progression-free survival, pharmacokinetics, and immunogenicity. Seventy patients were enrolled (n = 29 monotherapy; n = 41 combination therapy); most received prior HER2-targeted agents (monotherapy, 93%; combination therapy, 95%). With monotherapy, 69% of patients had any-grade treatment-related AEs (TRAEs); 17% had grade ≥ 3 TRAEs. The most common any-grade TRAEs were diarrhea (41%) and infusion-related reactions (24%). With combination therapy, 98% of patients had any-grade TRAEs; 51% had grade ≥ 3 TRAEs. The most common any-grade TRAEs were diarrhea (68%) and fatigue (44%). Confirmed ORR was 32.1% (95% confidence interval [CI] 15.9–52.4) with monotherapy and 48.6% (95% CI 31.9–65.6) with combination therapy. In heavily pre-treated patients with HER2-expressing GEA, zanidatamab ± chemotherapy had a manageable safety profile and promising antitumor activity.
Similar content being viewed by others
Introduction
Human epidermal growth factor receptor 2 (HER2) is a validated biomarker that is overexpressed in approximately 20% of gastroesophageal adenocarcinomas (GEAs), including adenocarcinomas of the gastroesophageal junction (GEJ), stomach, and distal esophagus1,2,3. HER2-positivity, defined as immunohistochemistry (IHC) 3+ or IHC 2+ with gene amplification by fluorescence in situ hybridization (FISH)4,5, has largely been reported to be a negative prognostic factor for GEA, while also being predictive of response to HER2-targeted therapy6,7,8,9.
The HER2-targeted antibody trastuzumab with pembrolizumab in combination with chemotherapy is the preferred first-line treatment for patients with HER2-positive GEA and with tumors having a programmed death-ligand 1 combined positive score (CPS) ≥ 1, based on results from the KEYNOTE-811 phase 3 trial10,11,12,13. For patients with HER2-positive GEA and a CPS < 1, the preferred first-line treatment remains trastuzumab plus chemotherapy based on the phase 3 Trastuzumab for Gastric Cancer (ToGA trial)10,11,12,14. After progression on first-line therapy, trastuzumab deruxtecan, a HER2-targeted antibody-drug conjugate, is the preferred HER2-targeted therapy based on the results of the phase 2 DESTINY-Gastric01 and DESTINY-Gastric02 trials10,11,12,15,16. To date, other HER2-targeted agents have not demonstrated significant benefits in any treatment setting17,18. As approved HER2-targeted treatments for GEA are limited, there remains a need for novel therapies in HER2-positive GEA to improve outcomes for patients.
Zanidatamab is a humanized, bispecific, immunoglobulin G isotype 1-like antibody under clinical development for the treatment of HER2-expressing cancers, including HER2-positive GEA19. Zanidatamab has a unique mechanism of binding whereby it targets two distinct non-overlapping HER2 domains (extracellular domains 4 and 2)20. Its structure promotes binding in trans, enabling it to crosslink neighboring HER2 proteins, creating large clusters on the surface of the cell. Zanidatamab clustering leads to multiple mechanisms of action, including the induction of complement-dependent cytotoxicity and other immune-mediated effects (e.g., antibody-dependent cellular cytotoxicity [ADCC] and phagocytosis [ADCP]); the prevention of HER2 dimerization and intracellular signaling; and facilitating HER2 internalization and subsequent degradation20. Zanidatamab has demonstrated greater in vivo antitumor activity than trastuzumab in a high HER2-expressing (IHC 3+) xenograft model of gastric cancer20.
A first-in-human, phase 1, multi-part dose-escalation and expansion study (NCT02892123) evaluated zanidatamab in patients with advanced or metastatic HER2-expressing solid tumors, including GEA21. As previously reported, zanidatamab monotherapy was well tolerated and demonstrated encouraging antitumor activity in patients with HER2-expressing tumors, including biliary tract cancer and colorectal cancer21.
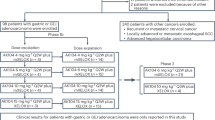
During dose-escalation (Part 1), no dose-limiting toxicities were observed, and the maximum tolerated dose was not reached17. The primary analysis of Part 1 and Part 2 (dose expansion) did not report data from patients with GEA. Here, we report the results in patients with previously treated HER2-expressing GEA (IHC 3+ or 2+, regardless of FISH status) from the zanidatamab monotherapy expansion cohort (Part 2) and the zanidatamab plus chemotherapy group (Part 3).
Results
Patient disposition and demographics
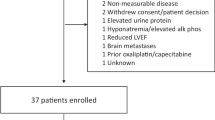
Patients were enrolled from September 1, 2016, to March 13, 2021. The data cutoff for this analysis was May 1, 2023. The cohort overview is shown in Fig. 1, which presents the recommended doses of zanidatamab as determined in the dose-escalation phase (Part 1) of the study21. In the zanidatamab monotherapy group, 29 patients had GEA, 28 had HER2-positive GEA and one had a HER2-status IHC 2+/FISH−. In the zanidatamab plus chemotherapy group, of 41 patients with GEA, 27 had HER2-positive GEA and 14 had IHC 2+/FISH−. Patients in the zanidatamab plus paclitaxel groups (n = 24 total) received zanidatamab 20 mg/kg (n = 11) or 25 mg/kg once every 2 weeks (Q2W) plus paclitaxel (n = 13). Patients in the zanidatamab plus capecitabine groups (n = 17 total) received zanidatamab 20 mg/kg Q2W (n = 6) or 30 mg/kg once every 3 weeks (Q3W) plus capecitabine (n = 11). At data cutoff, 64 of 70 (91.4%) total patients discontinued from the study (n = 29 [100%] in the monotherapy group; n = 35 [85.4%] in the combination therapy group). Among the six patients in the combination group who had not discontinued from the study at data cutoff, two had discontinued paclitaxel but were still receiving zanidatamab and four continued to receive combination therapy. The median (range) number of treatment cycles initiated in the monotherapy and combination therapy groups were 4 (1–24) months and 8 (1–60) months, respectively; in the combination therapy groups there was no difference in the number of treatment cycles between paclitaxel and capecitabine. The most common reason for treatment discontinuation was disease progression (69.0% in the monotherapy and 77.1% in the combination therapy group; Fig. 1). Median (range) duration of follow-up was 4.8 (0.1–24.0) months in the monotherapy and 7.4 (0.2–55.3) months in the combination therapy group.
Cap capecitabine, FISH fluorescent in situ hybridization, GEA gastroesophageal adenocarcinoma, HER2 human epidermal growth factor receptor 2, IHC immunohistochemistry, QW every week, Q2W every 2 weeks, Q3W every 3 weeks, Pac paclitaxel, PD progressive disease. aHER2-positive defined as IHC 3+ or IHC 2+/FISH+. bResponse evaluable analysis set.
Demographics and clinical characteristics are shown in Table 1 and Supplementary Table 2. The representativeness of study participants is presented in Supplementary Table 3. Overall, most patients who received zanidatamab monotherapy had gastric, followed by GEJ, then esophageal cancer. Most patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1 and stage IV disease at time of diagnosis. Patients received a median (range) of 2 (0–7) prior systemic therapies (one patient in the zanidatamab monotherapy group was enrolled without prior systemic therapy, which was documented as a protocol deviation). These baseline characteristics were similar in patients who received zanidatamab combination therapy. Almost all patients had received trastuzumab (93% in the monotherapy group, 95% in the combination therapy group).
Safety
In patients who received zanidatamab monotherapy, at least one treatment-emergent adverse event (TEAE) of any grade was reported in 97% (28/29) of patients; 52% (n = 15) had grade ≥ 3 TEAEs. In patients who received zanidatamab plus chemotherapy, at least one TEAE of any grade was reported in 100% of patients; 78% (n = 32) patients had grade ≥ 3 TEAEs. A summary of TEAEs in the monotherapy and combination groups is shown in Supplementary Table 4.
Overall, 69% (20/29) of patients who received zanidatamab monotherapy had a treatment-related adverse event (TRAE) of any grade. Most were grade 1 or 2, whereas 17% (5/29) of patients experienced grade 3 events. There were no grade 4 or 5 events. No patients discontinued zanidatamab due to a TRAE. The most common TRAEs in patients who received zanidatamab monotherapy were diarrhea, infusion-related reactions (IRRs), and fatigue (Table 2). Twelve patients experienced a TRAE of diarrhea. Only one case was grade 3 (this patient required hospitalization and had zanidatamab withheld temporarily). This was the only treatment-related serious adverse event (SAE) reported in the monotherapy group. Treatment-related adverse events of special interest (AESIs) included a grade 2 confirmed cardiac event of ejection fraction decreased in one patient that led to zanidatamab dose reduction from 20 mg/kg Q2W to 15 mg/kg Q2W on day 1 of cycle 3.
In the zanidatamab plus chemotherapy group, TRAEs were reported in 98% (40/41) of patients. Approximately one-half of these patients (21/41; 51.2%) experienced a grade ≥ 3 TRAE. No patients in this group discontinued zanidatamab due to a TRAE. Three patients received a dose reduction of zanidatamab due to TRAEs: two due to diarrhea, and one due to dehydration and fatigue. The most common TRAEs of any grade in patients receiving zanidatamab plus chemotherapy were diarrhea, fatigue, and alopecia (Table 2 and Supplementary Table 5). Treatment-related diarrhea was reported in 75% (18/24) of patients receiving zanidatamab plus paclitaxel and 59% (10/17) receiving zanidatamab plus capecitabine. Among all patients in the zanidatamab plus chemotherapy group, grade 3 diarrhea was reported by three patients in the zanidatamab 25 mg/kg Q2W plus paclitaxel group. None were considered serious; two resolved with the use of concomitant antidiarrheal medication and without dose modification, and one received concomitant antidiarrheal medication but was lost to follow-up after disease progression with the outcome of the diarrhea unknown. There were no cases of grade > 3 diarrhea. The most common grade ≥ 3 TRAEs were decreased neutrophil count and anemia, reported in 15% and 12% of patients, respectively. IRRs were reported in six (15%) patients (five patients receiving zanidatamab plus paclitaxel and one receiving zanidatamab plus capecitabine); all of the events were grades 1 or 2. The IRRs were related to zanidatamab in five patients; a dose interruption was required for four of these patients. Three patients in the zanidatamab plus chemotherapy group experienced a treatment-related SAE. Only one SAE was zanidatamab-related (grade 3 nausea). This adverse event (AE) was also combination anti-cancer therapy-related. Additional combination anti-cancer therapy-related SAEs included blood creatinine increased and pneumonitis. Zanidatamab-related AESIs included potential cardiac events in five patients (none met the criteria to be considered a confirmed cardiac event): peripheral oedema (n = 1; grade 1), cardiac failure (n = 2; both grade 1), and ejection fraction decreased (n = 2; one grade 1 and one grade 2). At the time of analysis, four of these cases were resolving or resolved, and the dose remained unchanged for all patients.
Efficacy
The response evaluable analysis included 28 patients with HER2-expressing GEA who received zanidatamab monotherapy and 37 patients who received zanidatamab plus single-agent chemotherapy. In the monotherapy group, confirmed objective response rate (cORR) (95% confidence interval [CI]) was 32.1% (15.9–52.4) (all partial response [PR]), with a median duration of response (DOR) (95% CI) of 6.7 (1.9–11.1) months (Table 3). Target lesion reduction was observed in most patients (17/25) (Fig. 2a). Kaplan–Meier (KM)-estimated median progression-free survival (PFS) (95% CI) was 3.6 (1.8–7.2) months (Fig. 2c; Table 3). Results were similar when including only patients with HER2-positive GEA in the analysis (the one patient who was not HER2-positive had a confirmed PR). Patients with GEA HER2 status of IHC 3+ had a longer median PFS than IHC 2+/FISH+ patients: 3.6 (95% CI 1.8–7.2) months and 1.8 (95% CI 1.2–7.7) months, respectively. Further response endpoints by HER2 status are presented in Supplementary Table 6. The response to treatment over time is presented in Fig. 2e.
Antitumor activity in patients with HER2-expressing as demonstrated by a Reduction in target lesions in patients treated with zanidatamab monotherapy (n = 25a), b reduction in target lesions in patients treated with zanidatamab in combination with chemotherapy regimens (n = 35b), c KM estimate of PFS in patients treated with zanidatamab monotherapy (n = 29), d KM estimate of PFS in patients treated with zanidatamab plus chemotherapy (n = 41), e response over time in patients treated with zanidatamab monotherapy (n = 28), and f response over time in patients treated with zanidatamab in combination with chemotherapy regimens (n = 37). CI confidence interval, cCR confirmed complete response, cPR confirmed partial response, CR complete response, E esophageal, FISH fluorescence in situ hybridization, G gastric, GEA gastroesophageal adenocarcinoma, HER2 human epidermal growth factor receptor 2, IHC immunohistochemistry, J gastroesophageal junction, KM Kaplan–Meier, MRI magnetic resonance imaging, NE not evaluable, PD progressive disease, PR partial response, PFS progression-free survival, Q2W every 2 weeks, Q3W every 3 weeks, SD stable disease, T trastuzumab, Trt treatment. Dotted lines in panels (a) and (b) indicate a 20% increase and 30% decrease in tumor size. aThree evaluable patients were excluded without post-baseline assessments: one patient died, one patient had clinical progression, and one patient had a new lesion leading to PD without any per protocol scheduled post-baseline imaging (at the end of cycle 1 the patient had a brain MRI due to clinical indication that identified a new lesion leading to PD). bTwo evaluable patients were excluded: both died without a post-baseline disease assessment. cWith the exception of one patient in Part 2, IHC status presented is based on the centralized assessment; noting that the local assessment was used for enrollment into the study. Source data are provided with this paper.
Of the 37 evaluable patients with HER2-expressing GEA in the combination therapy group, cORR (95% CI) was 48.6% (31.9–65.6) in 18 patients, with two complete responses (CRs) (5.4%) and 16 PRs (43.2%; Table 3); of 26 patients that were HER2-positive, the cORR (95% CI) was 50.0% (29.9–70.1), and of 11 patients with a HER2 status of IHC 2+/FISH−, the cORR (95% CI) was 45.5% (16.7–76.6). Further response endpoints by HER2 status in the combination therapy group are presented in Supplementary Table 6. Median (95% CI) DOR was 18.3 (5.6–not evaluable [NE]) months overall. Antitumor activity in patients treated with zanidatamab plus single-agent chemotherapy was similar irrespective of chemotherapy (paclitaxel or capecitabine; Supplementary Table 7). Most patients (29/35) had a decrease in tumor size, irrespective of the chemotherapy regimen received (Fig. 2b). KM-estimated median PFS (95% CI) was 7.3 (5.4–11.5) months (Fig. 2d; Table 3). Patients with GEA HER2 status of IHC 3+ had a longer median PFS than IHC 2+/FISH+ and IHC 2+/FISH− patients: 7.7 (95% CI 5.4–NE) months, 5.4 (95% CI 0.1–20.1) months, and 3.0 (95% CI 1.4–10.1) months, respectively. The response to treatment over time is presented in Fig. 2f.
Discussion
This phase 1 study evaluated the safety and efficacy of zanidatamab across multiple HER2-expressing cancers, with results of these cohorts reported previously. Here, we have focused specifically on the outcomes in patients with advanced unresectable or metastatic GEA. The population included patients who had received multiple prior treatments, most of which involved HER2-directed therapies. In this population, zanidatamab, administered as monotherapy or in combination with either paclitaxel or capecitabine, demonstrated promising clinical activity and a tolerable AE profile.
Beyond the recognized clinical benefits observed with trastuzumab in the first-line setting, there are limited HER2-targeted treatment options to further improve outcomes for patients with advanced HER2-positive GEA. The oral tyrosine kinase inhibitor lapatinib did not show benefit in terms of OS in the primary efficacy population, although PFS and response outcomes were improved18. Similarly, the addition of pertuzumab to trastuzumab and chemotherapy did not significantly improve OS in patients with HER2-positive metastatic gastric or GEJ cancer compared with placebo (17.5 and 14.2 months, respectively)17. Contrary to the experience in breast cancer, continuation of HER2-blockade after initial progression has not clearly demonstrated benefit for the majority of patients with GEA. However, in the phase 2 DESTINY-Gastric01 trial, trastuzumab deruxtecan demonstrated improved ORR (51% vs. 14%) and median OS (12.5 vs. 8.4 months) compared with physician’s choice of chemotherapy in patients with HER2-expressing, locally advanced, or metastatic gastric or GEJ cancer with disease progression following trastuzumab-containing therapy15. Similar efficacy was found in the phase 2 DESTINY-Gastric02 trial16. In addition, a phase 2 study of 50 patients with previously treated HER2-positive advanced gastric or GEJ cancer and treated with trastuzumab combined with ramucirumab and paclitaxel reported median PFS and OS of 7.1 and 13.6 months, respectively, and safety profiles consistent with previous reports22.
In this phase 1, multi-part trial, zanidatamab monotherapy and in combination with single-agent chemotherapy had a manageable safety profile with the majority of TRAEs being grade 1-2; the most common TRAEs were diarrhea and IRRs in the monotherapy group, and diarrhea and fatigue in the combination therapy group. Diarrhea induced by treatment in cancer patients is common, and a systematic review and meta-analysis have suggested that the use of HER2-targeted agents in patients with cancer significantly increases the risk of developing all-grade diarrhea23. AEs of diarrhea in this study were managed with antidiarrheal agents as needed and usually did not require dose modification. For patients receiving zanidatamab in combination with chemotherapy, there were additional AEs reported compared with patients receiving zanidatamab alone, yet the additional AEs were typical of chemotherapy, were of low severity, and were for the most part easily manageable.
The safety findings demonstrated here with zanidatamab are largely similar to the safety profiles observed with other HER2-targeted therapies in GEA, including trastuzumab-based and pertuzumab-based combination therapies14,17,24,25. Additionally, anti-HER2 therapies have been linked to an increased risk of interstitial lung disease, including pneumonitis, as well as cardiotoxicity26,27. However, there were limited occurrences of these events observed in this study; one confirmed and five unconfirmed cardiac events, plus zero and one pneumonitis in the monotherapy and combination groups, respectively. Although it should be noted that these other studies mostly used the HER2-targeted treatment as first-line therapy, compared with the current study of patients who had received multiple prior treatments.
In this study, treatment with zanidatamab monotherapy or with chemotherapy exhibited encouraging antitumor activity (cORR, 32.1% and 48.6%, respectively) in the total population, which includes both HER2-positive, per American Society of Clinical Oncology (ASCO)/College of American Pathologist guidelines, and patients with a HER-status of IHC 2+/FISH−. These cORR results were comparable when repeating the analysis with only the patients with HER2-positive GEA in each group. While the majority of the total population was HER2-positive, there were comparable cORR results for patients who were HER2-positive and those who were IHC 2+/FISH− (50.0% and 45.5%, respectively) in the zanidatamab plus chemotherapy group. The median DOR in this study was 6.7 months with monotherapy and 18.3 months with combination chemotherapy. Previous studies in patients with HER2-positive GEA treated with trastuzumab plus chemotherapy in the first-line setting reported an objective response rate ranging from 47 to 52% and median DOR between 6.9 and 10.6 months14,24,25. Antitumor activity of zanidatamab plus chemotherapy in the current study of patients with HER2-expressing GEA who had received previous treatments, with a median of two prior lines of systemic therapy (including trastuzumab)28, is especially promising, given that the DOR for the combination group observed here was longer than prior reports of DOR for first-line treatment in GEA; although it should be noted that this study had a small sample size in a single-arm experience. The relatively high level of antitumor activity in the context of prior HER2-targeted therapy may be due to zanidatamab having multiple mechanisms of action. In addition to strong blockade of HER2 signaling, zanidatamab’s mechanisms of action include immune-mediated ADCC and ADCP20. These effects may contribute to its therapeutic efficacy and potentially support combination treatment with immunotherapy agents. Furthermore, as zanidatamab, but not trastuzumab ± pertuzumab, also induces potent complement-dependent cytotoxicity in preclinical studies, it may contribute to lymphocyte independent antitumor activity20.
This phase 1 basket study has several limitations. The small sample sizes limited the generalizability of the study findings, and restricted the ability to perform analysis based on the line of treatment. Centralized assessment of HER2 status was not available for all patients; repeat HER2 testing by evaluation of fresh or archival tumor for central review of HER2 status was not mandatory for patients with prior HER2-directed therapy, and as such local assessment was used for some patients. Notably, almost all patients had received trastuzumab, indicating a high HER2-positive rate using local assays. However, discrepancies in HER2 status were observed in some patients initially enrolled based on local assessment but later evaluated centrally, likely due to variability in testing methodologies, tumor heterogeneity, and sample handling differences. Furthermore, as this study was conducted before the regulatory approval of trastuzumab deruxtecan, only a few patients had received it in prior treatment; however, the efficacy of zanidatamab in patients with HER2-expressing solid tumors (including GEA previously treated with trastuzumab deruxtecan) will be evaluated in the phase 2 DiscovHER PAN-206 trial (NCT06695845). Finally, objective responses were determined by investigator assessment; central review of imaging was not performed.
In conclusion, the favorable safety profile and antitumor activity of zanidatamab observed in this study of patients with HER2-expressing GEA provide further support that zanidatamab is well tolerated and has encouraging activity in patients with advanced, HER2-expressing tumors who have progressed after standard therapies, including HER2-targeted agents. These encouraging results support ongoing and future development of zanidatamab as a therapeutic option for patients with advanced GEA. Ongoing phase 2 studies aim to provide further support for zanidatamab plus combination chemotherapy earlier in the treatment pathway in advanced HER2-positive (IHC 3+ or IHC 2+ with ISH+) gastric/GEJ adenocarcinoma (NCT04276493)29, as well as HER2-expressing GEA (IHC 3+ or 2+ with or without gene amplification), biliary tract cancer (IHC 3+ with or without gene amplification; or IHC 0, 1+, or 2+ with gene amplification), and colorectal cancer (IHC 3+ with or without gene amplification; or IHC 0, 1+, or 2+ with gene amplification) (NCT03929666)30. In addition, a phase 3 trial assessing zanidatamab with chemotherapy with or without tislelizumab (a programmed cell death protein 1 inhibitor) in patients with advanced/metastatic HER2-positive (IHC 3+ or IHC 2+ with ISH+) GEA (NCT05152147) is ongoing19.
Methods
Study design and patients
This study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. The study protocol and all amendments were approved by an independent ethics committee or institutional review board at each study site. The study protocol was previously published as part of the supplement for the publication reporting the primary analysis21 and is included as Supplementary Note 1 in the Supplementary Information. Data for each patient were recorded on a case report form. Data collection was completed for each patient who signed an informed consent form and underwent any screening assessment. All patients provided written informed consent and were enrolled from September 1, 2016, to March 13, 2021. This study was registered on ClinicalTrials.gov under the identifier NCT02892123, with the registration submitted on September 1, 2016.
This was a phase 1, three-part, multicenter, dose-escalation and expansion study designed to evaluate the safety, tolerability, and antitumor effects of zanidatamab in solid tumors (Supplementary Fig. 1). Part 1 was a standard 3 + 3 dose-escalation phase to identify maximum tolerated dose, optimal biological dose, or recommended doses of zanidatamab monotherapy in any HER2-expressing solid tumor21. Parts 2 and 3 aimed to characterize the safety, tolerability, and antitumor activity of the zanidatamab doses identified in Part 1. Part 2 evaluated zanidatamab as monotherapy in various tumor types (including GEA) and Part 3 assessed zanidatamab in combination with other chemotherapy for breast cancer and GEA. Patients were not randomized or blinded to treatment because there was no comparator arm in this study.
Eligible patients were adults (aged ≥18 years) with locally advanced (unresectable) and/or metastatic HER2-expressing solid tumors that had progressed after receipt of all therapies known to confer clinical benefit (unless ineligible to receive a specific therapy); patients were not required to complete a specific number of prior therapies as eligibility criteria varied across participating countries due to differences in payer and insurance coverage for prior treatments. Patients with GEA were required to have a HER2 status of IHC 3+ or IHC 2+/FISH+ (collectively: HER2-positive, per ASCO guidelines10) or IHC 2+/FISH−. In Part 2 of the study patients were enrolled based on central HER2 assessment, whereas in Part 3 patients were enrolled based on central or local HER2 assessments.
In Part 3, patients with GEA must have received ≥ 1 prior systemic chemotherapy regimen, and those with HER2-positive GEA were required to have received prior trastuzumab. All patients must have had measurable disease per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.131, and provided a fresh or archival tumor sample for central review of HER2 status. Archival tissue must have been taken within 6 months prior to enrollment and with no intervening HER2-targeted therapy. Additionally, all patients were required to have adequate hepatic, renal, and cardiac left ventricular function, and an ECOG PS of 0 or 1. Key exclusion criteria included any clinically important lung or heart disease, prolonged QTc interval, untreated brain metastases, or clinical evidence of leptomeningeal disease.
Exclusion criteria included: treatment with experimental therapies or cancer therapy not otherwise specified within 4 weeks of zanidatamab dosing, or treatment with anthracyclines (within 90 days), HER2-targeted therapies (including, but not limited to, trastuzumab, pertuzumab, lapatinib, or trastuzumab emtansine within 3 weeks) before first zanidatamab dose, or prior taxane treatment (specific to patients with GEA in the zanidatamab 25 mg/kg Q2W plus paclitaxel group); untreated brain metastases or clinically assessed leptomeningeal disease; major surgery or radiotherapy within 3 weeks of zanidatamab dosing; pregnancy or breastfeeding; history of life-threatening hypersensitivity to monoclonal antibodies or to recombinant proteins or excipients in drug formulation; any other cancer within 3 years before first zanidatamab dosing (except for contralateral breast cancer or other adequately treated cancers); uncontrolled renal, liver, or pancreatic disease; peripheral neuropathy (> grade 2); clinically significant interstitial lung disease or cardiac disease; known active hepatitis B or C or known infection with human immunodeficiency virus; use of corticosteroids administered at doses equivalent to > 15 mg per day of prednisone within 2 weeks of first zanidatamab dosing unless otherwise approved by the study medical monitor; corrected QT interval by Fridericia > 450 ms; and cancer therapy-related toxicity that remained unresolved to grade ≤ 1 (except alopecia, neuropathy [resolved to grade ≤ 2], and congestive heart failure, which must have been grade ≤ 1 in severity at time of occurrence and completely resolved). Patients with a history of noncompliance to medical regimens or those unwilling or unable to comply with the protocol were also ineligible to participate in the study.
Study treatment
All patients with GEA in Part 2 received zanidatamab 10 mg/kg once weekly (QW) or 20 mg/kg Q2W (Supplementary Fig. 1). In Part 3, patients with GEA received zanidatamab 20 mg/kg or 25 mg/kg Q2W plus paclitaxel (80 mg/m2 QW for weeks 1, 2, and 3 of each 4-week cycle); or zanidatamab 20 mg/kg Q2W plus one of two capecitabine dose regimens (2000 mg twice daily [BID] for 7 days in weeks 1 and 3 of a 4-week cycle; or 1000 mg/m2 BID on days 1–14 of a 21-day cycle); or zanidatamab 30 mg/kg Q3W plus capecitabine (1000 mg/m2 BID on days 1–14 of a 21-day cycle) (Supplementary Fig. 1; Supplementary Table 1). The selection of the combination agent in Part 3 was dependent on the patient’s prior treatment history and availability of slots in each treatment group. When more than one option was available, selection of treatment was based on investigator discretion. Premedication for potential IRRs was mandatory and included administration of corticosteroids, antihistamines, and paracetamol (acetaminophen) 30–60 min before zanidatamab infusion.
Outcomes
The primary study objective in Parts 2 and 3 was to characterize the safety and tolerability of zanidatamab monotherapy or in combination with selected anticancer agents, and thus the primary study endpoints for Parts 2 and 3 were assessment of AEs, SAEs, and deaths; frequencies of dose-limiting toxicities; zanidatamab and chemotherapy dose modifications; laboratory values; electrocardiogram abnormalities; ECOG PS; and echocardiogram/multiple gated acquisition scan assessment for an estimate of ejection fraction.
Secondary endpoints for Parts 2 and 3 were assessment of cORR (defined as the percentage of patients with confirmed CR or confirmed PR per RECIST v1.1); disease control rate (DCR; defined as the percentage of patients with CR, PR, or stable disease); and PFS; (defined as time from the first dose of study treatment to the date of documented disease progression, clinical progression, or death from any cause); and pharmacokinetics and immunogenicity. The pharmacokinetic and immunogenicity data were included as part of a population analysis and published previously in a standalone manuscript32.
Determination of HER2 status
HER2 status for patients with GEA was determined using IHC or FISH in accordance with College of American Pathologists/American Society for Clinical Pathology/ASCO guidelines for the assessment of HER2 in patients with GEA4,5, and using either a fresh biopsy or archived formalin-fixed, paraffin-embedded (FFPE) sample. HER2 assessment was first done locally for study enrollment, followed by a subsequent retrospective centralized review (USC Norris Comprehensive Cancer Center Laboratory, Los Angeles, USA) on FFPE biopsies collected ≤ 6 months prior to enrollment. For the analysis described here, HER2 status was based on the centralized assessment; in one case where this was not available, the local assessment was used.
Assessments
AEs were monitored throughout the study and up to 30 days after study drug discontinuation. AE severity was graded according to the National Cancer Institute-Common Terminology Criteria for Adverse Events, version 4.03. AESIs were defined per study protocol as IRRs, absolute decreases of ≥ 10 percentage points below baseline left ventricular ejection fraction (LVEF), symptomatic heart failure, and confirmed cardiac events (a subset of potential cardiac events identified in the broad cardiac failure standardized Medical Dictionary for Regulatory Activities queries that were clinically reviewed and determined to be consistent with cardiac events of absolute decrease in LVEF of ≥ 10 percentage points from pretreatment baseline and absolute value < 50%, and/or grade ≥ 2 heart failure). TEAEs were defined as events with an onset during or after receipt of the first dose of study treatment (zanidatamab or study-specified anticancer therapy) and ≤ 30 days after the last dose. TRAEs were defined as TEAEs assessed by the investigator as either “related” or with unknown relationship to zanidatamab or study-specified anticancer therapy.
Antitumor activity was evaluated by computed tomography and/or magnetic resonance imaging of the chest, abdomen, and pelvis plus additional areas of known or suspected tumor involvement (e.g., brain, extremities) at baseline, every 8 weeks during treatment and at end of treatment (unless the previous scan was performed ≤ 4 weeks before end of treatment). Tumor responses were evaluated by investigator assessment according to RECIST v1.131.
Statistical analyses
A power analysis was not done because sample size target enrollment was selected based on clinical rather than statistical consideration. Sample sizes of 6–46 patients with HER2-positive GEA and 6–15 patients with HER2 status IHC 2+/FISH− were planned for Part 2. In Part 3, sample sizes of approximately 15 patients in each of the paclitaxel-containing regimen groups were planned; 6–12 patients were planned for the zanidatamab plus capecitabine groups.
The safety analysis set included all patients with GEA who received at least one dose of zanidatamab in Parts 2 and 3. Efficacy outcomes, other than PFS, were analyzed in the response-evaluable analysis set, which included all patients with GEA with at least one measurable target lesion at baseline per RECIST v1.1 who had at least one post-baseline disease assessment or discontinued study treatment due to death or clinical progression. As per the statistical analysis plan, PFS was evaluated in the safety analysis set. For PFS analysis, patients with GEA who were alive without disease progression at the time of analysis were censored at the time of their last tumor assessment, or cycle 1 day 1 if there was no post-baseline tumor assessment or no clinical progression. If disease progression occurred after missing two consecutive disease assessments, patients were censored at the time of their last assessment before disease progression, and if new anticancer treatment was started before disease progression, patients were censored at the time of their last assessment prior to the date of new treatment.
All analyses were descriptive and conducted using SAS version 9.4. Binomial Clopper-Pearson 95% CIs were calculated for response outcomes. KM plots and estimates of the quartiles and their corresponding 95% CIs were computed for time-to-event outcomes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant data are provided with the manuscript and supporting files. The study protocol was previously published as part of the supplement for the publication reporting the primary analysis21 and is included as Supplementary Note 1 in the Supplementary Information. Jazz Pharmaceuticals has established a process to review requests from qualified external researchers for data from Jazz-sponsored clinical trials in a responsible manner that includes protecting patient privacy, assurance of data security and integrity, and furthering scientific and medical innovation. External researchers may submit requests for data generated from Jazz Pharmaceuticals-sponsored clinical trials. Requests must be for data owned by Jazz Pharmaceuticals from completed trials where the product/indication is approved in the US or EU. Requests will be reviewed based on factors including, but not limited to, Jazz’s ability to share the requested data, qualifications of the researcher, legitimacy of the research purpose and scientific merit. Additional details on Jazz Pharmaceuticals data sharing criteria and process for requesting access can be found at: https://www.jazzpharma.com/science/clinical-trial-data-sharing. Source data are provided with this paper.
References
Grieb, B. C. & Agarwal, R. HER2-Directed Therapy in Advanced Gastric and Gastroesophageal Adenocarcinoma: Triumphs and Troubles. Curr. Treat. Options Oncol. 22, 88 (2021).
Van Cutsem, E. et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18, 476–484 (2015).
Rice, T. W., Ishwaran, H., Ferguson, M. K., Blackstone, E. H. & Goldstraw, P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J. Thorac. Oncol. 12, 36–42 (2017).
Bartley, A. N. et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Am. J. Clin. Pathol. 146, 647–669 (2016).
Bartley, A. N., Washington, M. K., Ismaila, N. & Ajani, J. A. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline Summary From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. J. Oncol. Pr. 13, 53–57 (2017).
Li, H. et al. Relationship between HER2 overexpression and long-term outcomes of early gastric cancer: a prospective observational study with a 6-year follow-up. BMC Gastroenterol. 22, 238 (2022).
Baykara, M. et al. Clinical Significance of HER2 Overexpression in Gastric and Gastroesophageal Junction Cancers. J. Gastrointest. Surg. 19, 1565–1571 (2015).
Kurokawa, Y. et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer 18, 691–697 (2015).
Jorgensen, J. T. & Hersom, M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J. Cancer 3, 137–144 (2012).
Shah, M. A. et al. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J. Clin. Oncol. 41, 1470–1491 (2023).
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed 1 May 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Esophageal and Esophagogastric Junction Cancers, V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed 1 May 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.
Janjigian, Y. Y. et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 402, 2197–2208 (2023).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Shitara, K. et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Van Cutsem, E. et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 24, 744–756 (2023).
Tabernero, J. et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 19, 1372–1384 (2018).
Hecht, J. R. et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC-A Randomized Phase III Trial. J. Clin. Oncol. 34, 443–451 (2016).
Tabernero, J. et al. HERIZON-GEA-01: Zanidatamab + chemo +/- tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 18, 3255–3266 (2022).
Weisser, N. E. et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat. Commun. 14, 1394 (2023).
Meric-Bernstam, F. et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 23, 1558–1570 (2022).
Kim, C. G. et al. Trastuzumab Combined With Ramucirumab and Paclitaxel in Patients With Previously Treated Human Epidermal Growth Factor Receptor 2-Positive Advanced Gastric or Gastroesophageal Junction Cancer. J. Clin. Oncol. 41, 4394–4405 (2023).
Li, J. Diarrhea With HER2-Targeted Agents in Cancer Patients: A Systematic Review and Meta-Analysis. J. Clin. Pharm. 59, 935–946 (2019).
Rivera, F. et al. Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother. Pharm. 83, 1175–1181 (2019).
Janjigian, Y. Y. et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 600, 727–730 (2021).
Ma, Z. et al. Interstitial lung disease associated with anti-HER2 anti-body drug conjugates: results from clinical trials and the WHO’s pharmacovigilance database. Expert Rev. Clin. Pharm. 15, 1351–1361 (2022).
Copeland-Halperin, R. S., Liu, J. E. & Yu, A. F. Cardiotoxicity of HER2-targeted therapies. Curr. Opin. Cardiol. 34, 451–458 (2019).
Roviello, G. et al. Immune Checkpoint Inhibitors in Pre-Treated Gastric Cancer Patients: Results from a Literature-Based Meta-Analysis. Int. J. Mol. Sci. 21, 448 (2020).
Lee, K. W. et al. Zanidatamab (zani), a HER2-targeted bispecific antibody, in combination with chemotherapy (chemo) and tislelizumab (TIS) as first-line (1L) therapy for patients (pts) with advanced HER2-positive gastric/gastroesophageal junction adenocarcinoma (G/GEJC): Preliminary results from a phase 1b/2 study. J. Clin. Oncol. 40, 4032–4032 (2022).
Elimova, E. et al. Zanidatamab + chemotherapy as first-line treatment for HER2-expressing metastatic gastroesophageal adenocarcinoma (mGEA). J. Clin. Oncol. 41, 347–347 (2023).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Proctor, J. R., Gartner, E. M., Gray, T. E. & Davies, R. H. Population pharmacokinetics of zanidatamab, an anti-HER2 biparatopic antibody, in patients with advanced or metastatic cancer. Cancer Chemother. Pharm. 90, 399–408 (2022).
Acknowledgements
We thank the patients, their families, and caregivers for participating in this study and all investigators and site personnel who contributed to the study. Medical writing, under the direction of the authors, was provided by Sharon Smalley, BSc (Hons), and Angharad Morgan, PhD, CMPP, on behalf of CMC Affinity, a division of IPG Health Medical Communications, funded by Jazz Pharmaceuticals, in accordance with Good Publication Practice (GPP 2022) guidelines.
Author information
Authors and Affiliations
Contributions
F.M.-B. contributed to study conceptualization. FM-B and LY contributed to formal analysis of the data. F.M.-B., S.Y.R., E.H., Y.-K.K., D.L.H., S.I., K.-W.L., J.L., M.B., D.-Y.O., J.C., R.A.G., J.A.A., and E.E. collected the data and provided resources. L.Y. contributed to development of the methodology. F.M.-B. contributed to the study validation. F.M.-B. and L.Y. contributed to the study visualization. All authors contributed to the writing, reviewing, and editing of the manuscript and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
F.M.-B. reports consulting or personal fees from AbbVie, Aduro BioTech, Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F Hoffman-La Roche, Genentech, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, OrigiMed, PACT Pharma, Parexel International, Pfizer, Samsung Bioepis, Seagen, Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks, and Chugai; advisory board participation for Black Diamond, Biovica, Eisai, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology, Seagen, Silverback Therapeutics, Spectrum Pharmaceuticals, and Zentalis; and research support, unrelated to this work and paid to institution, from Aileron Therapeutics, AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences, Curis, CytomX Therapeutics, Daiichi-Sankyo, Debiopharm International, eFFECTOR Therapeutics, Genentech, Guardant Health, Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology, and Taiho Pharmaceutical. S.Y.R. reports consulting fees from MSD Oncology, Daiichi-Sankyo, Eisai, LG Chem, Eutilex, Indivumed, AstraZeneca, Ono Pharmaceutical, Amgen, Aadi, and Toray Industries; speaker fees from Eisai, MSD Oncology, Bristol Myers Squibb/Ono Pharmaceutical, Amgen, Daiichi-Sankyo/UCB Japan, and AstraZeneca; and research funding from Company: MSD Oncology, Bristol Myers Squibb, Eisai, Roche/Genentech, ASLAN Pharmaceuticals, Sillajen, Bayer, Daiichi-Sankyo, Lilly, AstraZeneca, BeiGene, Zymeworks, Astellas Pharma, Indivumed, and Amgen. E.H. has received research funding (to her institution) from AbbVie, Acerta Pharma, Accutar Biotechnology, ADC Therapeutics, Akeso Bio Australia, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond, Bliss BioPharmaceuticals, Boehringer Ingelheim, Cascadian Therapeutics, Clovis Oncology, Compugen, Context Therapeutics, Cullinan, Curis, CytomX, Daiichi-Sankyo, Dana Farber Cancer Institute, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Eli Lilly, Ellipses Pharma, Elucida Oncology, EMD Serono, FujiFilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, InventisBio, Jacobio, Karyopharm, K-Group Beta, Kind Pharmaceuticals, Leap Therapeutics, Loxo Oncology, Lycera, Mabspace, Macrogenics, MedImmune, Mersana, Merus, Millennium, Molecular Templates, Novartis, Nucana, Olema, OncoMed, Onconova Therapeutics, Oncothyreon, ORIC Pharmaceuticals, Orinove, Orum Therapeutics, Pfizer, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Prelude Therapeutics, Profound Bio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicine, Rgenix, Roche/Genentech, Seagen, Sermonix Pharmaceuticals, Shattuck Labs, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Zenith Epigenetics, and Zymeworks; and consulting/advisory role fees from AstraZeneca, Daiichi-Sankyo, Ellipses Pharma, Gilead Sciences, Greenwich LifeSciences, Janssen, Jazz Pharmaceuticals, Lilly, Loxo, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Pfizer, Relay Therapeutics, Roche/Genentech, Seagen, Stemline Therapeutics, Theratechnologies, Tubulis, Verascity Science, and Zentalis Pharmaceuticals. Y.-K.K. reports consulting fees from ALX Oncology, Zymeworks, Amgen, Novartis, Macrogenics, Daehwa, Blueprint, Surface Oncology, Bristol Myers Squibb, MSD, and Roche. D.L.H. declares no competing interests. S.I. reports consulting fees from MSD Oncology, Daiichi-Sankyo, Eisai, LG Chem, Eutilex, Astellas Pharma, Indivumed, AstraZeneca, Ono Pharmaceutical, Amgen, Aadi, and Toray Industries; speakers’ fees from Eisai, MSD Oncology, Bristol Myers Squibb/Ono Pharmaceutical, Amgen, Daiichi-Sankyo/UCB Japan, and AstraZeneca; and research funding from Company: MSD Oncology, Bristol Myers Squibb, Roche/Genentech, ASLAN Pharmaceuticals, Sillajen, Bayer, Daiichi-Sankyo, Lilly, AstraZeneca, BeiGene, Zymeworks, Astellas Pharma, Indivumed, and Amgen. K.-W.L. received grants for the present manuscript from Zymeworks (to his institution for conducting clinical trials); and also received grants from ABLBIO, ALX Oncology, Amgen, Astellas, AstraZeneca, BeiGene, Bolt Therapeutics, Daiichi-Sankyo, Elevar Therapeutics, Exelixis, Genome & Company, Green Cross Corp, Idience, InventisBio, Leap Therapeutics, Macrogenics, MedPacto, Merck KGaA, Metafines, MSD, Oncologie, Ono Pharmaceutical, Pharmacyclics, Roche, Seagen, Taiho Pharmaceutical, Trishula Therapeutics, and Y-BIOLOGICS (to his institution for conducting clinical trials outside the submitted work); received honoraria from Astellas, Boryung, Daiichi-Sankyo, Ono Pharmaceutical, and Sanofi-Aventis; and has participated on a data safety monitoring board or advisory board for ALX Oncology and Metafines. J.L. declares no competing interests. M.B. declares no competing interests. Do-Youn Oh has participated in advisory boards for Abbvie, ASLAN, Arcus Biosciences, Astellas, AstraZeneca, Bayer, Basilea, BeiGene, BMS/Celgene, Eutilex, Genentech/Roche, Halozyme, IQVIA, J-Pharma, LG Chem, Merck Serono, Mirati Therapeutics, MSD, Novartis, Taiho, Turning Point, Yuhan, and Zymeworks; and has received research grants from Array, AstraZeneca, BeiGene, Eli Lilly, Handok, MSD, Novartis, and Servier. J.C. declares no competing interests. R.A.G. has participated in advisory boards for AAA/Novartis, Amgen, Apobiologix, Astellas, AstraZeneca, BMS, Eisai, Ipsen, Merck, Pfizer, and Taiho; has received speaker fees from AAA, Amgen, Astellas, BMS, Eisai, Ipsen, Merck AZ, and Pfizer; and has received independent education grants from Apobiologix, Ipsen, and Pfizer. J.A.A. has received honoraria from Acrotech Biopharma, Aduro Biotech, Amgen, Astellas Pharma, AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, DAVA Pharmaceuticals, Fresenius Kabi, Gilead Sciences, GRAIL, Lilly, Merck, Novartis, Oncotherics, SERVIER, and Zymeworks; has participated in consulting or advisory roles for the American Cancer Society, Amgen, Arcus Biosciences, Astellas Pharma, BeiGene, Bristol Myers Squibb, Geneos, Gilead Sciences, Insys Therapeutics, Merck, Novartis, Servier, and Vaccinogen; received research Funding from Amgen, Astellas Pharma (Inst), Bristol Myers Squibb, Daiichi-Sankyo, Delta-Fly Pharma, MedImmune, Merck, Gilead Sciences, Lilly/ImClone, Novartis, ProLynx, Roche/Genentech, Taiho Pharmaceutical, Takeda, and Zymeworks. L.Y. is employed by Jazz Pharmaceuticals. R.O. was employed by Jazz Pharmaceuticals at the time of this study. E.E. is a consultant for Abbvie, Adaptimmune, Astellas, BeiGene, BMS, Daiichi-Sankyo, Jazz, Natera, Novartis, Viracta Tx, and Zymeworks; has received grant/research support from Amgen, Arcus Biosciences, AstraZeneca, BMS, Bold Therapeutics, Jazz, and Zymeworks; is a steering committee member for AstraZeneca and Jazz; and has a family member who is employed by MerckVaccines.
Peer review
Peer review information
Nature Communications thanks Harshabad Singh, who co-reviewed with Mike Wang; Angelica Petrillo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meric-Bernstam, F., Rha, S.Y., Hamilton, E. et al. Zanidatamab monotherapy or combined with chemotherapy in HER2-expressing gastroesophageal adenocarcinoma: a phase 1 trial. Nat Commun 16, 4293 (2025). https://doi.org/10.1038/s41467-025-59279-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59279-z