Abstract
Rice is one of the most important staple food and model species in plant biology, yet its quantitative proteomes are largely uncharacterized. Here we quantify the relative protein levels of over 15,000 genes across major rice tissues using a tandem mass tag strategy followed by intensive fractionation and mass spectrometry. We identify tissue-specific and tissue-enriched proteins that are linked to the functional specificity of individual tissues. Proteogenomic comparison of rice and Arabidopsis reveals conserved proteome expression, which differs from mammals in that there is a strong separation of species rather than tissues. Notably, profiling of N6-methyladenosine (m6A) across the rice major tissues shows that m6A at untranslated regions is negatively correlated with protein abundance and contributes to the discordance between RNA and protein levels. We also demonstrate that our data are valuable for identifying novel genes required for regulating m6A methylation. Taken together, this study provides a paradigm for further research into rice proteogenome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw data of m6A-seq2 have been deposited into the National Center for Biotechnology Information Gene Expression Omnibus with accession number GSE229334. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE and iProx partner repository with the dataset identifiers PXD041188 and PXD052005. Source data are provided with this paper.
References
Nobuta, K. et al. An expression atlas of rice mRNAs and small RNAs. Nat. Biotechnol. 25, 473–477 (2007).
Wang, L. et al. A dynamic gene expression atlas covering the entire life cycle of rice. Plant J. 61, 752–766 (2010).
Zhao, L. et al. Integrative analysis of reference epigenomes in 20 rice varieties. Nat. Commun. 11, 1–16 (2020).
Chen, M. X. et al. Full-length transcript-based proteogenomics of rice improves its genome and proteome annotation. Plant Physiol. 182, 1510–1526 (2020).
Eraslan, B. et al. Quantification and discovery of sequence determinants of protein-per-mRNA amount in 29 human tissues. Mol. Syst. Biol. 15, e8513 (2019).
Giansanti, P. et al. Mass spectrometry-based draft of the mouse proteome. Nat. Methods 19, 803–811 (2022).
Mergner, J. et al. Mass-spectrometry-based draft of the Arabidopsis proteome. Nature 579, 409–414 (2020).
Jiang, L. et al. A quantitative proteome map of the human body. Cell 183, 269–283 e219 (2020).
He, P. C. & He, C. m6A RNA methylation: from mechanisms to therapeutic potential. EMBO J. 40, e105977 (2021).
Frye, M., Harada, B. T., Behm, M. & He, C. RNA modifications modulate gene expression during development. Science 361, 1346–1349 (2018).
Wang, X. et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014).
Wang, X. et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015).
Pertea, G. & Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res https://doi.org/10.12688/f1000research.23297.2 (2020).
Dierks, D. et al. Multiplexed profiling facilitates robust m6A quantification at site, gene and sample resolution. Nat. Methods 18, 1060–1067 (2021).
Wong, C. E. et al. Shaping the landscape of N6-methyladenosine RNA methylation in Arabidopsis. Plant Physiol. 191, 2045–2063 (2023).
Liu, J. et al. Landscape and regulation of m6A and m6Am methylome across human and mouse tissues. Mol. Cell 77, 426–440 e426 (2020).
Yu, Q. et al. RNA demethylation increases the yield and biomass of rice and potato plants in field trials. Nat. Biotechnol. 39, 1581–1588 (2021).
Shen, L., Ma, J., Li, P., Wu, Y. & Yu, H. Recent advances in the plant epitranscriptome. Genome Biol. 24, 43 (2023).
Liang, Z. et al. Epigenetic modifications of mRNA and DNA in plants. Mol. Plant 13, 14–30 (2020).
Bassal, M. et al. Reshaping of the Arabidopsis thaliana proteome landscape and co-regulation of proteins in development and immunity. Mol. Plant 13, 1709–1732 (2020).
Wang, P. et al. A large-scale proteogenomic atlas of pear. Mol. Plant 16, 599–615 (2023).
Liu, Q. et al. Transcriptional landscape of rice roots at the single-cell resolution. Mol. Plant 14, 384–394 (2021).
Wisniewski, J. R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009).
Li, J. et al. TMTpro reagents: a set of isobaric labeling mass tags enables simultaneous proteome-wide measurements across 16 samples. Nat. Methods 17, 399–404 (2020).
Su, D. et al. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat. Protoc. 9, 828–841 (2014).
Shen, L. et al. N6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev. Cell 38, 186–200 (2016).
Jiang, Y. H., Cheng, K., Zhao, X. B. & Ouyang, Y. D. Isolation and transformation of rice protoplasts. BioProtoc. Bio-101, e1010125 (2018).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Wang, M., Jiang, L. & Snyder, M. P. AdaTiSS: a novel data-adaptive robust method for identifying tissue specificity scores. Bioinformatics 37, 4469–4476 (2021).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Feng, J., Liu, T., Qin, B., Zhang, Y. & Liu, X. S. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 7, 1728–1740 (2012).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Wei, X. et al. A quantitative genomics map of rice provides genetic insights and guides breeding. Nat. Genet. 53, 243–253 (2021).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Acknowledgements
This work was supported by Biological Breeding Major Projects (2023ZD04076 to Z.L.), National Natural Science Foundation of China (3217040580 to Z.L.), The Agricultural Science and Technology Innovation Program (ASTIP) (to Z.L.), Central Public Interest Scientific Institution Basal Research Fund (to Z.L.), STI2030 Major Projects (2022ZD0401904 to S.-T.L.), the National Research Foundation, Singapore, under its Competitive Research Programme (NRF-CRP22-2019-0001 to H.Y.) and Central Laboratory of Biotechnology Research Institute, CAAS.
Author information
Authors and Affiliations
Contributions
S.-T.L., Y.K. and Z.L. conceived and designed the study. Y.K., T.-Y.Z., L.L., C.L. and X.L. performed the experiments. S.-T.L., Y.K., Y.Z., H.H., Z.H., X.Z., Y.L., M.X., L.Z., C.M., F.W., X.G., B.Y., H.Y. and Z.L. analysed data. S.-T.L., H.Y. and Z.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Alisdair Fernie, Natalie Clark and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
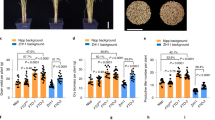
Extended Data Fig. 1 Quantitative proteomic map of rice tissues.
a, Tandem mass tag (TMT) 16-plex- and MS3-based mass spectrometry quantitative proteomics workflow. Proteins were extracted and digested and labeled with TMT 16-plex, respectively. 16 samples were mixed and followed with HPLC separation. The samples were intensively fractionated into 24 fractions and analyzed by HPLC-MS/MS, followed with computational analysis. Mega ref, two mixed samples used to normalize the protein expression and assess batch effects. Three biological replicates were performed for each tissue. b, SDS-PAGE profile of proteins from 14 rice tissues. The representative ones from three biological replicates were shown. c, Pie charts showing the percentage of proteins identified by one or multiple peptides and grouped by UniProt protein evidence annotations (PE1-4). Numbers in brackets refer to the number of identified proteins, along with the number of unique genes they represent. d, Comparison of the number of identified protein between this study and earlier studies with top 10 highest identified protein numbers in rice. e, Proportion of different types of novel protein coding events (NPCEs) in rice. NPCE type was analyzed by GFFcompare software. c, intron compatible. j, multi-exon with at least one junction match. k, containment of reference. o, other name strand overlap with reference exons. u, unknown or intergenic. x, exonic overlap on the opposite strand. f, Heatmap showing the expression profile of tissue-specific NPCEs. Each line indicates one NPCE. g, Distribution of relative protein abundance (log2intensity) before normalization across samples. h, Distribution of relative protein abundance (log2intensity) after quantile normalization, which remove potential batch effect across samples. n = 15,174. The boxplots show the median (line) and 25th and 75th percentiles of the data. Whiskers extended 1.5 times the interquartile range. Outliers were omitted from the boxplot but were included in the analysis.
Extended Data Fig. 2 Quantitative proteome analysis across rice tissues.
a, Heatmap showing Pairwise global Pearson’s correlation analysis of transcriptome for all rice tissues. Core, gene with TPM > 1. Non-core, gene with TPM < 1. b, Heatmap showing Pairwise global Pearson’s correlation analysis of proteome for all rice tissues. Core, gene with TPM > 1. Non-core, gene with TPM < 1. c, Heatmap showing pattern of expression of tissue-specific proteins. If TS score of a protein is greater than 4 in a tissue and is at least 1.5 higher than the protein’s TS scores in any other tissue, this protein is considered as tissue specific. d, Number of tissue-enriched and tissue-specific genes at the transcriptome level in rice. See Methods section for the definition of tissue-enriched and tissue-specific genes. e, KEGG analysis of a subset of proteins enriched in rice atypical photosynthetic tissues stem and spike neck. BH method was used for multiple hypothesis adjustment.
Extended Data Fig. 3 Proteome comparative analysis between Arabidopsis and rice, and PTR in rice.
a, Heatmap showing pairwise global transcriptome correlation coefficients between Arabidopsis and rice for the matching tissues. b, PCA of the Arabidopsis and rice matching tissues showing a predominant clustering of the transcriptome by species. Each tissue is represented by a colour. c. Distribution of median PTR across rice tissues. d, GO enrichment analysis for genes with lower PTRs. GO analysis and adjusted p values calculation were performed by the R package clusterProfiler. BH method was used for multiple hypothesis adjustment.
Extended Data Fig. 4 Quantitative m6A methylomes across rice tissues.
a, m6A-seq2 workflow. The mRNAs were fragmented, dephosphorylated and ligated with different barcode 3′ adaptor. Samples were mixed in a tube and immunoprecipitated with m6A anti-body, then m6A-IP samples and input RNAs were subjected to reverse transcription, 5′ adaptor ligation and sequencing. Three biological replicates were performed for each tissue. b, Boxplot comparing m6A intensity across rice tissues. m6A intensity for each region was calculated as (RPKMIP/RPKMInput), while the total m6A intensity of a tissue was defined as accumulated peak intensity for all m6A peaks in the corresponding tissue. The boxplots show the median (line) and 25th and 75th percentiles of the data. Whiskers extended 1.5 times the interquartile range. Dots indicate outliers. c, Odds ratio of rice trait QTLs enriched in m6A peaks normalized to size-matched random input regions in corresponding tissues. d, Representative Integrative Genomics Viewer (IGV) views of tissue-enriched m6A across rice tissues. Red box indicates the tissue-enriched m6A peak. e, Cumulative distribution of changes in gene expression between m6A modified and non-m6A genes in different rice tissues, respectively. p values were calculated by two-sided Mann-Whitney test and all are significant (p < 2.2e−16). f, Boxplot comparison of expression levels between m6A modified and non-m6A genes in different rice tissue, respectively. The boxplots show the median (line) and 25th and 75th percentiles of the data. Whiskers extended 1.5 times the interquartile range. Outliers were omitted from the boxplot but were included in the analysis. p values were calculated unpaired two-sided Mann-Whitney U test. Gene numbers were listed below the p values.
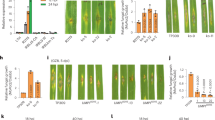
Extended Data Fig. 5 m6A contributes to the protein abundance in rice, and identify MED18 as a novel player that affect m6A in plants.
a, Comparison of Spearman’s correlation (protein abundance and RNA expression level) between m6A modified genes on different position (5′UTR; CDS; 3′UTR) and non-m6A genes in 14 rice tissues. b, Comparison of Spearman’s correlation (protein abundance and RNA expression level) among m6A modified genes on different position in 14 rice tissues. All, m6A on UTR or CDS; CDS, m6A only on CDS; UTR, m6A only on UTR. c, Heatmap showing relative protein abundance of the potential m6A writers, easers and readers across rice tissues. The protein family information was shown on the right. d, Scatter plots showing the correlations between calculated overall m6A level and the protein abundance of potential m6A regulators. e, Protein abundance of MED18 across rice tissues. f, Schematic of the comparison between rice wild type (WT), med18-3 and med18-4. The arrows indicate the CRISPR-Cas9 targeted sites. g, Sequence comparison between rice WT MED18, med18-1, med18-2, med18-3 and med18-4. h, Phenotype comparing between rice 3-week-old WT and med18 seedlings. Scale bar, 1cm. i, Bar plot comparing the length of root (left) and shoot (right) between rice WT, med18-3 and med18-4 plants. p values were calculated by two-sided paired Student’s t test. n = 29. Error bars, mean ± SD. j, LC-MS/MS quantification of the m6A/A ratio in mRNA of 3-week-old rice WT, med18-3 and med18-4 plants. p values were calculated by two-sided paired Student’s t test. n = 3 biological replicates. Error bars, mean ± SD.
Supplementary information
Supplementary Tables 1–11
Supplementary Table 1. Rice materials for TMT 16-plex labelled proteome and m6A-seq2. Supplementary Table 2. List of number of identified proteins in rice proteomic studies. Supplementary Table 3. List of NPCEs. Supplementary Table 4. The relative abundance of proteins for each tissue. Supplementary Table 5. The TS score for each protein across tissues. Supplementary Table 6. List of tissue-enriched and tissue-specific proteins. Supplementary Table 7. The mRNA TPM value across tissues. Supplementary Table 8. List of genes with the top 2,000 PTR. Supplementary Table 9. m6A level determined by LC–MS/MS for each tissue. Supplementary Table 10. List of m6A peaks for each rice tissue. Supplementary Table 11. Primers and probes used in this study.
Source data
Source Data Fig. 1
Unprocessed gels for Fig. 7c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, ST., Ke, Y., Zhu, Y. et al. Mass spectrometry-based proteomic landscape of rice reveals a post-transcriptional regulatory role of N6-methyladenosine. Nat. Plants 10, 1201–1214 (2024). https://doi.org/10.1038/s41477-024-01745-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-024-01745-5