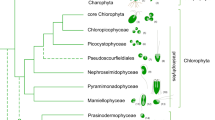
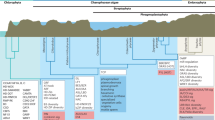
Abstract
The emergence of land plants was a pivotal development in Earth history. It has been postulated that the evolutionary transition from freshwater streptophyte algae to land plants, or the canalization of plant meiosis, was completed during the Middle Ordovician (~460 Ma). However, the absence of undisputed streptophyte algal fossils (for example, Charophyceae) earlier than the late Silurian (~425 Ma) has obscured this link between streptophyte algae and land plants. Here we describe a marine Charophyceae fossil, Tarimochara miraclensis gen. et sp. nov., from early and middle Katian (Late Ordovician, ~453–449 Ma) marine limestones in northwestern China. This discovery demonstrates that at least some species of Charophyceae inhabited shallow normal marine environments at that time. Moreover, these early Charophyceae show that some key morphological innovations associated with an evolutionary transition between streptophyte algae and land plants had occurred before the early Katian. This provides crucial evidence relevant to the origins of land plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data analysed in this Article, including the phylogenetic datasets, are available as part of the Article, Extended Data Figs. 1–10, Supplementary Information and Supplementary Table 1.
References
Morris, J. L. et al. The timescale of early land plant evolution. Proc. Natl Acad. Sci. USA 115, E2274–E2283 (2018).
Servais, T. et al. Revisiting the great Ordovician diversification of land plants: recent data and perspectives. Palaeogeogr. Palaeoclimatol. Palaeoecol. 534, 109280 (2019).
Strother, P. K. & Foster, C. A fossil record of land plant origins from charophyte algae. Science 373, 792–796 (2021).
Lenton, T. et al. Earliest land plants created modern levels of atmospheric oxygen. Proc. Natl Acad. Sci. USA 113, 9704–9709 (2022).
Spencer, C. et al. Composition of continental crust altered by the emergence of land plants. Nat. Geosci. 15, 735–740 (2022).
Karol, K. G., McCourt, R. M., Cimino, M. T. & Delwiche, C. F. The closest living relatives of land plants. Science 294, 2351–2353 (2001).
McCourt, R. M., Delwiche, C. F. & Karol, K. G. Charophyte algae and land plant origins. Trend Ecol. Evol. 19, 661–666 (2004).
Becker, B. & Marin, B. Streptophyte algae and the origin of embryophytes. Ann. Bot. 103, 999–1004 (2009).
Wodniok, S. et al. Origin of land plants: do conjugating green algae hold the key? BMC Evol. Biol. 11, 104 (2011).
Delwiche, C. F. & Cooper, E. D. The evolutionary origin of a terrestrial flora. Curr. Biol. 25, 899–910 (2015).
Hall, J. D. & McCourt, R. in Handbook of the Protists (eds Archibald, J. M. et al.) 135–163 (Springer, 2017).
McCourt, R. M., Karol, K. G., Hall, J. D., Casanova, M. T. & Grant, M. C. in Handbook of the Protists (eds Archibald J. M., Simpson, A. & Slamovits, C. H.) 165–183 (Springer, 2017).
Cook, M. E. & Graham, L.E. in Handbook of the Protists (eds Archibald J. M., Simpson, A. & Slamovits, C. H.) 185–204 (Springer, 2017).
De Vries, J. & Archibald, J. M. Plant evolution: landmarks on the path to terrestrial life. N. Phytol. 217, 1428–1434 (2018).
Nishiyama, T. et al. The Chara genome: secondary complexity and implications for plant terrestrialization. Cell 174, 448–464 (2018).
Cheng, S. F. et al. Genomes of subaerial zygnematophyceae provide insights into land plant evolution. Cell 179, 1057–1067 (2019).
Donoghue, P. & Paps, J. Plant evolution: assembling land plants. Curr. Biol. 30, R64–R91 (2019).
Leebens-Mack, J. H. et al. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574, 679–685 (2019).
Jiao, C., Iben, S., Sun, X., Sun, H. & Rose, J. K. C. The Penium margaritaceum genome: hallmarks of the origins of land plants. Cell 181, 1097–1111 (2020).
Donoghue, P. C. J. et al. The evolutionary emergence of land plants. Curr. Biol. 31, R1281–R1298 (2021).
Harris, B. J. et al. Divergent evolutionary trajectories of bryophytes and tracheophytes from a complex common ancestor of land plants. Nat. Ecol. Evol. 6, 1634–1643 (2022).
Bowles, A. M. C. et al. The origin and early evolution of lands. Trends Plant Sci. 28, 312–329 (2023).
Bowman, J. L. The origin of a land flora. Nat. Plants 8, 1352–1369 (2022).
Strother, P. K. & Taylor, W. A. in Transformative Paleobotany (eds Kings, M. et al.) 3–20 (Elsevier, 2018).
Strother, P. K. & Taylor, W. A. A fossil record of spores before sporophytes. Diversity 16, 428 (2024).
Feist, M. et al. in Treatise on Invertebrate Paleontology, Part C, Protista 1 Vol. 1 (ed. Kaesler R. L.) 1–168 (Geological Society of America and Univ. Kansas Press, 2005).
Soulié-Märsche, I. & García, A. Gyrogonites and oospores, complementary viewpoints to improve the study of the charophytes (Charales). Aquat. Bot. 120, 7–17 (2015).
Ishchenko, T. A. & Ishchenko, A. A. in Sistématika i êvolutsia drevnik rastenii Ukraini (ed. Teslenko, J. V.) 21–32, pl.5-6 (Naukowa Dumka, Kiev., 1982).
Martín-Closas, C. & Diéguez, C. Charophytes from the lower Cretaceous of the Iberian Ranges (Spain). Palaeontology 41, 1133–1152 (1998).
Anadon, P., Utrilla, R. & Vazquez, A. Use of charophyte carbonates as proxy indicators of subtle hydrological and chemical changes in marl lakes: example from the Miocene Bicorb Basin, eastern Spain. Sediment. Geol. 133, 325–347 (2000).
Climent-Domènech, H., Martín-Closas, C. & Salas, R. Charophyte-rich microfacies in the Barremian of the eastern Iberian Chain (Spain). Facies 55, 387–400 (2009).
Villalba-Breva, S. & Martín-Closas, C. A characean thallus with attached gyrogonites and associated fossil charophytes from the Maastrichtian of the eastern Pyrenees (Catalonia, Spain). J. Phycol. 47, 131–143 (2011).
Pełechaty, M. et al. The significance of Chara vegetation in the precipitation of lacustrine calcium carbonate. Sedimentology 60, 1017–1035 (2013).
Kawahata, C., Yamamuro, Y. & Shiraiwa, Y. Changes in alkaline band formation and calcification of corticated charophyte Chara globularis. SpringerPlus 2, 85 (2013).
Berger S. & Kaever M. J. Dasycladales: An Illustrated Monograph of a Fascinating Algal Order 1–247 (Georg Thieme Verlag, 1992)
Gómez-Poot, J. M., Espinoza-Avalos, J. & Jiménez-Flores, S. G. Vegetative and reproductive characteristics of two species of Batophora (Chlorophyta, Dasycladaceae) from Chetumal Bay, Quintana Roo, Mexico. Bot. Mar. 45, 189–195 (2002).
Shen, Y. F. & Neuweiler, F. Taphocoenoses and diversification patterns of calcimicrobes and calcareous algae, Ordovician, Tarim Basin, China. Can. J. Earth Sci. 53, 702–711 (2016).
Feist, M., Liu, J. & Tafforeau, P. New insights into Paleozoic charophyte morphology and phylogeny. Am. J. Bot. 92, 1152–1160 (2005).
Feist, M., Nazik, A., Schindler, E., Wehrmann, A. & Yaln, M. N. Distribution and palaeoecology of charophyte floras in Devonian coastal environments of the central Taurides (Turkey). Palaeobiodiv. Palaeoenviron.99, 353–366 (2019).
Liu, L. J., Wu, Y. S., Jiang, H. X., Wu, N. Q. & Jia, L. Q. Paleoenvironmental distribution of Ordovician calcimicrobial associations in the Tarim Basin, Northwest China. Palaios 32, 462–489 (2017).
Liu, L. J. et al. Diversity and systematics of Middle–Late Ordovician calcified cyanobacteria and associated microfossils from Ordos Basin, North China. J. Paleontol. 95, 1–23 (2021).
Riding, R. Calcareous Algae and Stromatolites (Springer, 1991).
Wray, J. L. Calcareous Algae 1–185 (Elsevier, 1977).
Liu, L. J. et al. Calcareous algae from the Upper Ordovician Lianglitage Formation in the Tarim Basin, Xinjiang, China. Acta Micropalaeontol. Sin. 29, 18–38 (2012).
Kenrick, P. & Li, C. S. An early, non-calcified, dasycladalean alga from the Lower Devonian of Yunnan Province, China. Rev. Palaeobot. Palynol. 100, 73–88 (1998).
Coneva, V. & Chitwood, D. H. Plant architecture without multicellularity: quandaries over patterning and the soma-germline divide in siphonous algae. Front. Plant Sci. 6, 287 (2015).
Riding, R. & Fan, J. S. Ordovician calcified algae and cyanobacteria, northern Tarim Basin subsurface, China. Palaeontology 44, 783–810 (2001).
Zhang, Y. Y. et al. Calcified dasycladaleans from the Upper Ordovician in the Ordos Basin, China. Acta Micropalaeontol. Sin. 37, 228–237 (2020).
Conkin, J. E. & Conkin B. M. Late Silurian (Ludlovian) charophyte Moellerina laufeldi n. sp., from the Karma Beds of the Isle of Gotland, Sweden. Univ. Louisville Notes Paleontol. Strat. J, 1–21,6 pl. (1992).
Mamet, B., Roux, A., Lapointe, M. & Gauthier, L. Algues ordoviciennes et siluriennes de l’Ile Anticosti (Québec, Canada). Rev. Micropaléontol. 35, 211–248 (1992).
Harholt, J., Moestrup, Ø. & Ulvskov, P. Why plants were terrestrial from the beginning? Trends Plant Sci. 21, 96–101 (2016).
Gunnarsson, K. & Jónsson, S. Benthic marine algae of Iceland: revised checklist. Cryptogam. Algol. 23, 131–158 (2000).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Guo, Y. R., Zhao, Z. Y., Shi, X. Y. & Gao, J. R. Sequence stratigraphy of the Ordovician system in the Ordos Basin. Acta Sedimentol. Sin. 32, 44–60 (2014).
Zhang, Y. D. et al. Ordovician integrative stratigraphy and timescale of China. Sci. China Earth Sci. 62, 61–88 (2019).
Bowman, J. L., Kohchi, T., Yamato, K. T., Jenkins, J. & Schmutz, J. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171, 287–304 (2017).
Strother, P. K. Systematics and evolutionary significance of some new cryptospores from the Cambrian of eastern Tennessee, USA. Rev. Palaeobot. Palynol. 227, 28–41 (2016).
Strother, P. K., Traverse, A. & Vecoli, M. Cryptospores from the Hanadir Shale Member of the Qasim Formation, Ordovician (Darriwilian) of Saudi Arabia: taxonomy and systematics. Rev. Palaeobot. Palynol. 212, 97–110 (2015).
Steemans, P. et al. Origin and radiation of the earliest vascular land plants. Science 324, 353 (2009).
Wellman, C. H., Osterloff, P. L. & Mohiuddin, U. Fragments of the earliest land plants. Nature 425, 282–285 (2003).
Edwards, D. & Feehan, J. Records of Cooksonia-type sporangia from late Wenlock strata in Ireland. Nature 287, 41–42 (1983).
Libertín, M., Kvacek, J., Bek, J., Zársky, V. & Storch, P. Sporophytes of polysporangiate land plants from the early Silurian period may have been photosynthetically autonomous. Nat. Plants 4, 269–271 (2018).
Acknowledgements
We thank Y. Dai for valuable suggestions concerning the identification of Charophyceae fossils; J. Fan for help in obtaining research materials; H. Jiang for help with the field work and core sample collection; X. Zhou and C. Wellman for valuable advice, L. Liang and L. Jia for comments on the paper; W. Zhuang for help with phylogenetic analysis; and J. Shen for help with fossil reconstruction. This work was supported by the National Key Research and Development Program of China (grant number 2023YFF0803601 to Z.Z.), the National Natural Science Foundation of China (grant numbers 42072127 to L.L., U22B6004 to W. Zhao, 41930319 to D. Fu and 41972143 to L. Jia) and the Key Core Technology Research Project of Changqing Oilfield Company (grant number KJZX2023-01 to W. Zhao).
Author information
Authors and Affiliations
Contributions
L.L. led the project. Y.W. led the field work, core sample collection, thin preparation and research into sedimentary facies. L.L. participated in field work and core sample collection. L.L. discovered the fossils, critically analysed the results and conceptualized the paper. L.L. and R.R. wrote the paper. L.L., R.R., J.H., Q.T., K.P., Z.Z., R.L., B.G., Y.W., H.H. and C.C. oversaw the results and discussion; L.L, J.H., Z.Z., R.L. and B.G. carried out the phylogenetic analysis; L.L. prepared the figures and supplementary information. All co-authors contributed to the interpretations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Björn Kröger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of transmitted polarized light and Cathode-luminescence (CL) observation of cortical cells of T. miraclensis.
a, c, Transmitted polarized light micrograph, showing transverse section of axis with surrounding cortical (cor.) cells, TZ16-14-73-70 (2). b, d, CL observation of the same field of view a and b, very dull cathodoluminescence of the encrustations of the cortical cells indicates relatively good preservation and minimal alteration by contemporaneous fluids. e, Transmitted polarized light micrograph, showing a longitudinal section through an axis with parallel cortical (cor.) cells, TZ16-14-73-70 (2). f. CL observation of the area of the yellow dashed outline in (e), very dull cathodoluminescence of the encrustations of cortical cells indicates relatively good preservation and minimal alteration by contemporaneous fluids.
Extended Data Fig. 2 Transverse, oblique, and longitudinal section views of well-preserved T. miraclensis thalli.
a-d, Transverse section views of axis and cortical (cor.) cells, No.TZ16-14-73-70(1) and TZ16-14-73-70 (2). e, Longitudinal and oblique section views of axis and cortical cells, No.TZ16-14-73-70(1). f-g, Transverse and longitudinal section views of axis and cortical cells, No. TZ16-14-73-70 (2), and TZ83-5220.87m, respectively.
Extended Data Fig. 3 Transverse, oblique, views of broken axis of T. miraclensis.
a, Transverse section of axis showing a curved chain of cortical (cor.) cells, No. TZ822-5720.75m. b, Transverse sections of axis, No. M401-33-29-22. c-d, Sections in various planes, M401-2414.06 m. e, Oblique axial section, No. TZ822-8-108-73. f, Sections in various planes, M401-2414.06 m. g, Oblique axial section, No. TZ826-5768.18m.
Extended Data Fig. 4 Dimensions of T. miraclensis.
a, External and internal diameters of cortical cells and axes based on 16 relatively well-preserved specimens. b, External diameters of axis and cortical cells show broad positive correlation, showing smaller and larger groups. c, Dimensions of probable utricle, including external width and wall thickness.
Extended Data Fig. 5 TIMA (TESCAN Integrated Mineral Analyzer) and XRF analysis of T. miraclensis. specimens and surrounding rock matrix.
a, Transmitted polarized light micrograph, showing that fossil specimens of T. miraclensis are lighter than the matrix, TZ16-14-73-70 (1).b, TIMA mineral mapping of a, showing T. miraclensis preserved as calcite and distinguished from surrounding matrix occupied by clay minerals. c, TIMA elemental mapping of a, showing that T. miraclensis is deficient Si, whereas the matrix is enriched in Si relatively. d, Transmitted light micrograph of T. miraclensis, TZ16-14-73-70 (2). e-g, XRF elemental mappings of d. e, Both matrix and thalli are poor in Si compared to clay minerals that fill the microcracks. f, Thalli of T. miraclensis are poor in K compared to matrix, the clay minerals filling microcracks are enriched in K. g, Both matrix and thalli are poor in Al compared to the clay minerals filling microcracks.
Extended Data Fig. 7 Stratigraphic and abundance distribution of T.miraclensis in platform interior boreholes of the Tarim Basin.
Facies divisions are based on ref. 40.
Extended Data Fig. 8 T. miraclensis in tidal flat and lagoonal facies, Lianglitag Formation, Tarim Basin.
a–d, Tidal flat; a, Fenestral micrite, containing sparse bioclasts including T. miraclensis (T) and echinoderms (ech.), No.TZ73-8-83-47. b, Fenestral micrite, No.TZ161-26-52-46. c, fenestral micrite, containing T. miraclensis broken ‘stem’ and probable utricle, TZ82. d, Packstone, containing several broken ‘stems’ of T. miraclensis, No.TZ73-4857.05. e, f, Lagoonal facies; e, micrite containing T. miraclensis, Tetradium (Tet.) (coralomorph), and Zonotrichites (Zon.) (calcified cyanobacterium), No.TZ161-30-50-39. f, Micrite containing Tarimochara and amsassiaceans (Ams.), No.TZ16-15-13-12. For recognition of tidal lagoonal facies, see ref. 40.
Extended Data Fig. 9 T. miraclensis in open platform and bank facies of the Lianglitag Formation, Tarim Basin.
a, Bioclastic grainstone containing T. miraclensis (T), corals (cor.), and the dasycladalean Dasyporella (Das.), TZ161-25-55-28. b, Bioclastic grainstone containing T. miraclensis, TZ822-9-5727.55. c, Open platform packstone containing T. miraclensis and abundant dasycladaleans: Vermiporella (Ver.) and Arthroporella (Art.), TZ70-6-66-21. d, Bioclastic grainstone, containing T. miraclensis, echinoderms (ech.), and bryozoans (bry.), TZ822-5727.55. e, Open platform packstone, containing T. miraclensis and unidentified bioclasts, TZ24-7-48-44. f, TZ70-9-58-48, Open platform packstone, containing T. miraclensis. g, Open platform packstone, containing dasycladaleans (das.) T. miraclensis, TZ161-32-52-19. For recognition of open platform and bank facies see ref. 40.
Extended Data Fig. 10 T. miraclensis in reef facies of the Lianglitag and Beiguoshan formations, Tarim and Ordos basins.
a, Reef framework formed by the dasycladacean Aphroporella (Aph.) and red algae, containing T. miraclensis, TZ42-2-49-21. b, Reef framework of Tetradium (coralomorph), Vermiporella (green alga), and Phacelophyton (calcified cyanobacterium), with T. miraclensis, TZ24-25. c, Reef framework of the dasycladalean Vermiporella (Ver.), containing T. miraclensis, LJP-6(2)-A. d, Reef framework with T. miraclensis, bryozoans, and echinoderms, TZ242-4546.82. e, Reef facies with the calcified cyanobacterial sheath Girvanella (Gir.), T. miraclensis bioclasts, and echinoderms, TZ822-5664.41. f, Coralomorph Tetradium (Tet.) reef framework with T. miraclensis, TZ24-7-48-44. Recognition of reef facies is based on refs. 40,41.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and discussion.
Supplementary Table 1
Dimensions and occurrences of Tarimochara in the thin sections.
Supplementary Data 1
Characteristic–taxon matrix in NEXUS format, 500,000 generations (statistical source data for Supplementary Fig. 1b).
Supplementary Data 2
Characteristic–taxon matrix in NEXUS format, 1,000,000 generations (statistical source data for Fig. 5b and Supplementary Fig. 1a).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Han, J., Zhang, Z. et al. Ordovician marine Charophyceae and insights into land plant derivations. Nat. Plants 11, 1116–1126 (2025). https://doi.org/10.1038/s41477-025-02003-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-025-02003-y