Abstract
Cold stress restricts root growth by disrupting stem cell activity in plants. C-REPEAT BINDING FACTORs (CBFs) are central regulators of cold signalling and also modulate root stem cell activity. While the receptor-like cytoplasmic kinase CRPK1 promotes CBF destabilization under cold stress, its regulatory partner remains unclear. Here we identify KINASE ON THE INSIDE (KOIN), a plasma-membrane-localized receptor-like kinase, as a crucial interactor of CRPK1. The loss of either KOIN or CRPK1 results in cold-insensitive root phenotypes, characterized by sustained primary root elongation and enhanced cortex cell proliferation via the 14-3-3–CBF3–SHR pathway. Under cold stress, KOIN undergoes endocytosis and is recycled back to the plasma membrane in a CRPK1-dependent manner. Although catalytically inactive, KOIN modulates CRPK1 protein levels and phosphorylation through a non-catalytic mechanism. These findings uncover a membrane-to-nucleus signalling module that integrates receptor trafficking with intracellular kinase activity to mediate cold-induced root growth inhibition in Arabidopsis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the article, the extended data figures or the Supplementary information. The biological materials used in this study are available from the corresponding author upon reasonable request. The Arabidopsis gene sequences referenced in this study can be accessed through the TAIR database (https://www.arabidopsis.org/) under the following accession numbers: KOIN (AT5G58300), CRPK1 (AT1G16670), 14-3-3λ (AT5G10450), 14-3-3κ (AT5G65430), CBF1 (AT4G25490), CBF2 (AT4G25470), CBF3 (AT4G25480) and SHR (AT4G37650). Source data are provided with this paper.
References
Zhang, J., Li, X. M., Lin, H. X. & Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 70, 753–780 (2019).
Zhu, J. et al. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 56, 727–736 (2015).
Fu, D. et al. Regulation of alternative splicing by CBF-mediated protein condensation in plant response to cold stress. Nat. Plants 11, 505–517 (2025).
Thomashow, M. F. PLANT COLD ACCLIMATION: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599 (1999).
Hong, J. H. et al. A sacrifice-for-survival mechanism protects root stem cell niche from chilling stress. Cell 170, 102–113.e114 (2017).
Shi, Y., Ding, Y. & Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 23, 623–637 (2018).
Jaglo-Ottosen, K. R., Gilmour, S. J., Zarka, D. G., Schabenberger, O. & Thomashow, M. F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280, 104–106 (1998).
Song, Y. et al. The direct targets of CBFs: in cold stress response and beyond. J. Integr. Plant Biol. 63, 1874–1887 (2021).
Jia, Y. et al. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. N. Phytol. 212, 345–353 (2016).
Perez-Garcia, P. et al. The cold-induced factor CBF3 mediates root stem cell activity, regeneration, and developmental responses to cold. Plant Commun. 4, 100737 (2023).
Helariutta, Y. et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101, 555–567 (2000).
Wang, C. et al. A conserved megaprotein-based molecular bridge critical for lipid trafficking and cold resilience. Nat. Commun. 13, 6805 (2022).
De Smet, I., Voß, U., Jürgens, G. & Beeckman, T. Receptor-like kinases shape the plant. Nat. Cell Biol. 11, 1166–1173 (2009).
Yang, T., Chaudhuri, S., Yang, L., Du, L. & Poovaiah, B. W. A calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. J. Biol. Chem. 285, 7119–7126 (2010).
Liu, Z. et al. Plasma membrane CRPK1-mediated phosphorylation of 14-3-3 proteins induces their nuclear import to fine-tune CBF signaling during cold response. Mol. Cell 66, 117–128.e5 (2017).
Rodriguez-Furlan, C., Campos, R., Toth, J. N. & Van Norman, J. M. Distinct mechanisms orchestrate the contra-polarity of IRK and KOIN, two LRR-receptor-kinases controlling root cell division. Nat. Commun. 13, 235 (2022).
Jones, A. M. et al. Border control—a membrane-linked interactome of Arabidopsis. Science 344, 711–716 (2014).
Murphy, A. S., Bandyopadhyay, A., Holstein, S. E. & Peer, W. A. Endocytotic cycling of PM proteins. Annu. Rev. Plant Biol. 56, 221–251 (2005).
Rodriguez-Furlan, C., Minina, E. A. & Hicks, G. R. Remove, recycle, degrade: regulating plasma membrane protein accumulation. Plant Cell 31, 2833–2854 (2019).
Geldner, N. et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59, 169–178 (2009).
Reiling, J. H. et al. A CREB3–ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens. Nat. Cell Biol. 15, 1473–1485 (2013).
Renault, L., Guibert, B. & Cherfils, J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature 426, 525–530 (2003).
Oruganty, K., Talathi, N. S., Wood, Z. A. & Kannan, N. Identification of a hidden strain switch provides clues to an ancient structural mechanism in protein kinases. Proc. Natl Acad. Sci. USA 110, 924–929 (2012).
Casamitjana-Martínez, E. et al. Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem maintenance. Curr. Biol. 13, 1435–1441 (2003).
Takeuchi, H. & Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531, 245–248 (2016).
Erwig, J. et al. Chitin-induced and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) phosphorylation-dependent endocytosis of Arabidopsis thaliana LYSIN MOTIF-CONTAINING RECEPTOR-LIKE KINASE5 (LYK5). N. Phytol. 215, 382–396 (2017).
Liebrand, T. W., van den Burg, H. A. & Joosten, M. H. Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 19, 123–132 (2014).
Nakajima, K., Sena, G., Nawy, T. & Benfey, P. N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311 (2001).
Winter, C. M. et al. SHR and SCR coordinate root patterning and growth early in the cell cycle. Nature 626, 611–616 (2024).
Gallagher, K. L., Paquette, A. J., Nakajima, K. & Benfey, P. N. Mechanisms regulating SHORT-ROOT intercellular movement. Curr. Biol. 14, 1847–1851 (2004).
Cui, H. et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 316, 421–425 (2007).
Achard, P. et al. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20, 2117–2129 (2008).
Peng, Y. et al. Differential phosphorylation of Ca2+-permeable channel CYCLIC NUCLEOTIDE-GATED CHANNEL20 modulates calcium-mediated freezing tolerance in Arabidopsis. Plant Cell 36, 4356–4371 (2024).
Mbengue, M. et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc. Natl Acad. Sci. USA 113, 11034–11039 (2016).
Postma, J. et al. Avr4 promotes Cf-4 receptor-like protein association with the BAK1/SERK3 receptor-like kinase to initiate receptor endocytosis and plant immunity. N. Phytol. 210, 627–642 (2016).
McNeil, P. L., Miyake, K. & Vogel, S. S. The endomembrane requirement for cell surface repair. Proc. Natl Acad. Sci. USA 100, 4592–4597 (2003).
McNeil, P. L. & Terasaki, M. Coping with the inevitable: how cells repair a torn surface membrane. Nat. Cell Biol. 3, E124–E129 (2001).
Reddy, A., Caler, E. V. & Andrews, N. W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106, 157–169 (2001).
Cao, Y. et al. The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife 3, e03766 (2014).
Gomez-Gomez, L., Bauer, Z. & Boller, T. Both the extracellular leucine-rich repeat ___domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13, 1155–1163 (2001).
Boller, T. & Felix, G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406 (2009).
Sun, Y. et al. Structural basis for flg22-induced activation of the Arabidopsis FLS2–BAK1 immune complex. Science 342, 624–628 (2013).
Stegmann, M. et al. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355, 287–289 (2017).
Schindelin, J., Rueden, C. T., Hiner, M. C. & Eliceiri, K. W. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol. Reprod. Dev. 82, 518–529 (2015).
Lv, J. et al. Reciprocal regulation between the negative regulator PP2CG1 phosphatase and the positive regulator OST1 kinase confers cold response in Arabidopsis. J. Integr. Plant Biol. 63, 1568–1587 (2021).
Ding, Y. et al. OST1-mediated BTF3L phosphorylation positively regulates CBFs during plant cold responses. EMBO J. 37, e98228 (2018).
Walter, M. et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438 (2004).
Furuta, Y. et al. Petal abscission is promoted by jasmonic acid-induced autophagy at Arabidopsis petal bases. Nat. Commun. 15, 1098 (2024).
Ding, Y. et al. CPK28–NLP7 module integrates cold-induced Ca2+ signal and transcriptional reprogramming in Arabidopsis. Sci. Adv. 8, eabn7901 (2022).
Zeng, R. et al. A natural variant of COOL1 gene enhances cold tolerance for high-latitude adaptation in maize. Cell 188, 1315–1329.e13 (2025).
Acknowledgements
We thank Y. Guo for providing seeds of marker lines and helpful discussion. We thank Y. Liu and S. Wang for help in the imaging. This work was supported by grants from the National Natural Science Foundation of China (no. 32230005) and Pinduoduo-China Agricultural University Research Fund (no. PC2023B01001) to S.Y.
Author information
Authors and Affiliations
Contributions
S.Y. designed the study. Xiuyue Zhang performed the experiments with the help of M.L. and Xiaoyan Zhang. Xiuyue Zhang, R.Z., Y.P., Y.S., X.W., W.Z. and Z.G. analysed the data. Xiuyue Zhang and S.Y. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of CRPK1 and KOIN.
a, Representative diagrams showing root growth measurement of 5-d-old seedlings grown at 22 °C and then either maintained at 22 °C or transferred to 4 °C for an additional 10 days. b, Root length of 5-d-old Col-0, crpk1-1 and crpk1-1 CRPK1 complementation seedlings grown at 22 °C and then kept at 22 °C for an additional 10 days. Data represent mean ± s.d. from one biological replicate (n = 20), representative of three independent experiments. c, Dual-membrane yeast two-hybrid assays showing negative controls. d, Schematic diagram of KOIN ___domain structure. SP, signal peptide; LRR, leucine-rich repeat; TM, transmembrane ___domain, CD, cytoplasmic ___domain. Amino acid positions at ___domain boundaries are indicated. e, In vitro pull-down assay showing the interaction between KOIN-CD with CRPK1. His-KOIN-CD and MBP-His-CRPK1 were immunoprecipitated with MBP beads and detected by anti-His immunoblotting. f, Co-IP assays in Nicotiana benthamiana showing interaction between KOIN–GFP and CRPK1–Myc. g, RT–qPCR analysis of KOIN transcript levels in Col-0 and koin-2 mutants. h, Immunoblot analysis of KOIN protein levels in Col-0 and koin-2 mutants using anti-KOIN antibody. Actin serves as a loading control in (g) and (h).
Extended Data Fig. 2 KOIN negatively regulates cold-induced root growth inhibition.
a,b, Root length of 5-d-old Col-0, koin-2 and koin-2 KOIN complementation seedlings grown at 22 °C and then maintained at 22 °C (a) or transferred to 4 °C (b) for 10 days. Data represent mean ± s.d. from one biological replicate (n = 20), representative of three independent experiments. c-h, Freezing tolerance of Col-0, koin mutants (c-e) and koin-2 KOIN complementation seedlings (f-h) under cold-acclimated conditions. Shown are representative seedling phenotypes (c,f), survival rates (d,g), and ion leakage (e,h). In (d-e) and (g-h), data are mean ± s.d. (n = 3 biological replicates). In (b, d-e) and (g-h), different letters indicate statistically significant differences (P < 0.05, one-way ANOVA with Tukey’s post hoc test). Exact P values are provided in the Supplementary Table 2.
Extended Data Fig. 3 Expression pattern and subcellular localization of KOIN.
a, Tissue specific expression of KOIN in Col-0 based on Genevestigator data. Expression potential (%) indicates average expression across tissues relative to the highest expression sample (set as 100%). b, GUS staining of pKOIN::GUS transgenic plants. c, RT–qPCR analysis of KOIN expression in Col-0 under cold treatment. Data represent mean ± s.d. (n = 3), representative of three independent experiments. d, Genevestigator-based expression patterns of KOIN under cold stress. e, Subcellular localization of KOIN-GFP in various tissues of koin-2 KOIN complementation plants. GFP, GFP fluorescence; PI, propidium iodide staining (red); merged images show overlays with chlorophyll, PI, or FM4-64. Scale bars: 10 μm (root tip, meristem zone, hypocotyl, epidermal cells); 5 μm (elongation zone, root hairs). f, KOIN-GFP localization in root tip. Scale bar, 20 μm. g, KOIN protein levels in Col-0 under cold stress. Anti-KOIN and anti-Actin antibodies were used. h, Identification of Super::KOIN-GFP (KOIN-OE) transgenic plants in Col-0 and crpk1-1 background. i-j, KOIN protein levels in Col-0 (i) or crpk1-1 (j) backgrounds under cold. In (h-j), anti-GFP and anti-Actin antibodies were used.
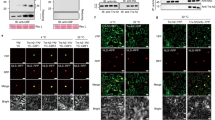
Extended Data Fig. 4 Re-localization of KOIN-GFP from BFA compartments to the plasma membrane is impaired in crpk1-1 seedlings.
a, Quantification of KOIN-GFP fluorescence intensity in Col-0 and crpk1-1 roots under cold treatment, corresponding to Fig. 3a–d. b, Relative fluorescence intensity of KOIN-GFP during recycling assays, corresponding to Fig. 3g, h. c, Number of BFA bodies per cell after sequential CHX, BFA + CHX, and BFA washout treatments as described in Fig. 3g, h. d, Quantitation of plasma membrane and cytosol fluorescence intensities of KOIN-GFP in Col-0 and crpk1-1 roots under the same treatments. Data are mean ± s.d. (n = 3 biological replicates). Different letters indicate significant differences (P < 0.05, one-way ANOVA with Tukey’s test). Exact P values are provided in the Supplementary Table 2.
Extended Data Fig. 5 Sequence analysis of KOIN.
a, Alignment of subdomains VIb and VII within the kinase catalytic cores of active RLKs (BRI1, FLS2, EFR, CRK7) and inactive RLKs (PRK4, PRK5, BIR2, SUB). b, Phylogenetic analysis of kinase catalytic core sequences in Arabidopsis. The tree was constructed using MEGA7 based on amino acid sequences of the kinase domains. c, Repeated in vitro phosphorylation assay showing CRPK1-mediated phosphorylation of KOIN, corresponding to Fig. 4d. Autoradiograph (top) and CBB staining (bottom) are shown. Phosphorylation intensities are normalized to wild-type KOIN (set as 1.0).
Extended Data Fig. 6 Identification of KOIN complemented transgenic plants.
a, Immunoblot analysis of KOIN, KOINS314A and koin-2 KOINS314E expression in koin background under the native promoter. KOIN was detected with anti-GFP antibodies; Actin serves as a loading control. b, In planta phosphorylation of wild-type and S314A mutant KOIN after cold treatment. Immunoprecipitated KOIN-GFP and KOINS314A-GFP were analyzed by immunoblotting using anti-GFP and anti-phosphoserine antibodies. c,d, Root length of 5-d-old Col-0, koin-2, koin-2 KOINS314A and koin-2 KOINS314E seedlings grown at 22 °C and then maintained at 22 °C (c) or transferred to 4 °C (d) for 10 days. Data represent mean ± s.d. (n = 20, one biological replicate), representative of three independent experiments. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA with Tukey’s post hoc test). Exact P values are provided in the Supplementary Table 2.
Extended Data Fig. 7 Phenotypic analysis of KOIN-OE and koin crpk1 transgenic lines expressing KOIN variants.
a, Subcellular localization of KOIN-GFP in root tips. Scale bar, 20 μm. b, Confocal images of median longitudinal sections of root tips; asterisks indicate the meristematic cortex. Scale bar, 20 μm. c, Quantification of cortical cell numbers shown in (b) at 0, 24, and 48 h of cold treatment. Data represent mean ± s.d. (n = 3 biological replicates). d, Root phenotypes of 5-d-old Col-0, koin-2, and KOIN-OE seedlings grown at 22 °C and then maintained at 22 °C or transferred to 4 °C for 10 days. Scale bar, 0.5 cm. e, Immunoblot analysis of KOIN protein levels in koin crpk1 mutants expressing KOIN, KOINS314A, or KOINS314E. Anti-GFP and anti-Actin antibodies were used for detection. f, Root length of 5-d-old Col-0, crpk1-1, koin-2 crpk1-1, and koin crpk1 mutants expressing KOIN variants. Seedlings were grown at 22 °C, and then maintained at 22 °C (top) or transferred to 4 °C (bottom) for 10 days. Data represent mean ± s.d. (n = 20, one biological replicate), representative of three independent experiments. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA with Tukey’s post hoc test). Exact P values are provided in the Supplementary Table 2. g,h, FM4-64 labeling of koin crpk1 double mutants expressing KOIN variants after cold treatment, showing KOIN-GFP recycling defects. Seedlings were incubated with FM4-64 for 5 min before imaging. Scale bar, 10 μm.
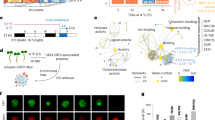
Extended Data Fig. 8 KOIN interacts with 14-3-3λ and modulates of CBF signaling under cold stress.
a, Co-IP assays in Arabidopsis protoplasts showing interaction between KOIN-GFP and Flag-HA-14-3-3λ. Proteins were detected using anti-GFP and anti-HA antibodies. b, In vitro pull-down assay showing the interaction between KOIN and 14-3-3λ. Anti-His antibodies were used for detection. c, BiFC assay in Arabidopsis protoplasts showing KOIN-14-3-3λ interaction at the plasma membrane. FM4-64 staining was used to visualize endosomes. BRI1–YFPN+14-3-3λ–YFPC served as a negative control. Negative control (BRI1-YFPN+14-3-3λ-YFPC) is shown. Scale bar: 5 μm. d,e, Transcript levels of CBFs (d) and their target genes (e) in Col-0, koin-2, koin-2 KOIN complementation lines under cold treatment. Expression is shown relative to untreated Col-0 (set as 1.0), with ACTIN2/8 as internal reference. Data represent mean ± s.d. (n = 3, one biological replicate), representative of three independent experiments. **P < 0.01 (one-way ANOVA with Tukey’s test). Exact P values are provided in the Supplementary Table 2.
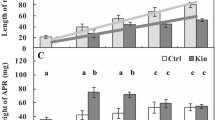
Extended Data Fig. 9 KOIN, CRPK1 and 14-3-3 s act upstream of CBFs to regulate cold-induced root growth inhibition.
a, Root length of 5-d-old Col-0, 14-3-3kλ, 14-3-3k/14-3-3λ, 14-3-3λ-OE, crpk1-1, koin-2, crpk1 14-3-3λ-OE and koin 14-3-3λ-OE seedlings grown at 22 °C, then either maintained at 22 °C or transferred to 4 °C for 10 days. b, Root length of 5-d-old Col-0, 14-3-3kλ, cbfs, 14-3-3kλ cbfs, crpk1-1, koin-2, crpk1 cbfs and koin cbfs mutants under the same conditions. Data represent mean ± s.d. (n = 20, one biological replicate), representative of three independent experiments. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA with Tukey’s post hoc test). Exact P values are provided in the Supplementary Table 2.
Supplementary information
Supplementary Table 1
List of primer sequences used in this study.
Supplementary Table 2
P values for Figs. 1, 2 and 4–6 and Extended Data Figs. 1, 2, 4 and 6–9.
Source data
Source Data Fig. 2
Unprocessed western blots for Fig. 2.
Source Data Fig. 4
Unprocessed western blots for Fig. 4.
Source Data Fig. 6
Unprocessed western blots for Fig. 6.
Source Data Extended Data Fig. 1
Unprocessed western blots and gels for Extended Data Fig. 1.
Source Data Extended Data Fig. 3
Unprocessed western blots for Extended Data Fig. 3.
Source Data Extended Data Fig. 5
Unprocessed western blots for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Unprocessed western blots for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Unprocessed western blots for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Unprocessed western blots for Extended Data Fig. 8.
Source Data Figs. 1, 2 and 4–6 and Extended Data Figs. 1–4 and 6–9
Statistical source data for Figs. 1, 2 and 4–6 and Extended Data Figs. 1–4 and 6–9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Li, M., Zhang, X. et al. A receptor–kinase cascade confers cold-induced root growth inhibition in Arabidopsis. Nat. Plants (2025). https://doi.org/10.1038/s41477-025-02034-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41477-025-02034-5