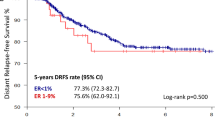
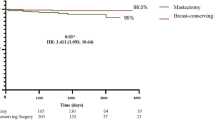
Abstract
We investigated whether tailored neoadjuvant therapy (chemotherapy [NCT] or endocrine therapy [NET]) guided by a 70-gene assay could improve breast-conserving surgery (BCS) rates among patients with ER-positive/HER2-negative breast cancer initially deemed ineligible for BCS. Of 130 prospectively enrolled patients (stage II–IIIA, across four Korean centers), 92 were analyzed. Patients classified as high genomic risk received NCT, while low-risk patients underwent NET (letrozole ± leuprolide for premenopausal women) for 16–24 weeks. The primary endpoint—achieving the surgeon-defined target tumor size for BCS—was reached in 69.6% (95% CI: 59.1–78.7%), significantly surpassing the predefined goal of 50.8% (p < 0.05). The actual overall BCS rate was 59.8% (64.7% NCT, 45.8% NET). Pathologic complete response occurred in 2.2%, exclusively in the NCT group. Thus, pretreatment genomic profiling effectively guided therapy selection, substantially increasing BCS eligibility while sparing low-risk patients unnecessary chemotherapy toxicity.
Similar content being viewed by others
Introduction
For individuals diagnosed with operable breast cancer, neoadjuvant chemotherapy (NCT) is established as a conventional approach, particularly for those expected to undergo adjuvant chemotherapy. Notably, compared to adjuvant chemotherapy, a significant advantage of NCT is its ability to increase the likelihood of breast-conserving surgery (BCS), which is a key benefit of this treatment strategy1,2,3. Evidence from a meta-analysis of 14 studies indicates a 29% reduction in mastectomy rates among NCT recipients compared with those in patients receiving adjuvant chemotherapy without compromising local control4. This underscores the pivotal role of NCT in enhancing breast conservation opportunities. However, the effectiveness of NCT in treating estrogen receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer remains under scrutiny, owing to the lower response and lower incidence of pathologic complete response (pCR) within this subgroup5.
Neoadjuvant endocrine therapy (NET) presents another option for ER+/HER2– breast cancer. Table 1 shows three NET studies with target population, interventions, and the results. In the P024 trial and IMPACT trial, the BCS conversion rate in postmenopausal women was significantly higher with aromatase inhibitors than tamoxifen6,7. In the STAGE trial, neoadjuvant anastrozole + goserelin showed significantly better overall tumor response than tamoxifen + goserelin8. Additionally, multigene assays have shown promising efficacy in predicting NCT or NET response in ER+/HER2– breast cancer. For instance, the 21-gene assay (Oncotype DX, Exact Sciences, USA) had a high recurrence score (RS) and was significantly associated with pCR in NCT patients9 and also inversely correlated with NET response10,11. Similarly, the 70-gene assay (MammaPrint; Agendia Inc., USA) used in this study has shown efficacy in identifying low-risk patients who may safely forgo chemotherapy and high-risk patients who can benefit from chemotherapy among patients with ER+/HER2– breast cancer12.
This study aimed to explore the potential of tailored treatments (NCT or NET) guided by the 70-gene assay in increasing BCS rates in patients with ER+/HER2– breast cancer (KBCSG016: PLATO trial).
Results
Of the 100 patients enrolled, seven patients who were categorized as high-risk based on genomic assessment declined neoadjuvant chemotherapy, and one patient who was lost to follow-up during therapy was excluded from the final analysis. The remaining 92 patients were finally included in the full analysis set. Among them, 68 patients (73.9%) were assigned to the genomic high-risk group (GH) and received NCT, whereas 24 (26.1%) patients were assigned to the genomic low-risk group (GL) and received NET (Fig. 1).
Patient’s characteristics and initial demographics are shown in Table 2. The median baseline tumor size on imaging was 3.7 cm (IQR 2.8–4.4 cm), with 87.0% of patients presenting as clinical T2 and 13.0% as clinical T3. The mean age of patients was 47.0 years in the GH group and 50.0 years in the GL group, with premenopausal patients comprising 64.7% and 62.5%, respectively. Histological high-grade tumors were significantly more prevalent in the GH group than in the GL group (20.6% vs. 0%, P = 0.007).
Table 3 summarizes the neoadjuvant treatment response and surgery results in each treatment group. The end-of-treatment (EOT) median tumor size on imaging was 2.2 cm (IQR 1.6–3.0 cm), with a median tumor size of 2.1 cm in the GH group and 2.4 cm in the GL group (P = 0.018). Clinically, 5.4% of patients exhibited complete response (CR), 73.9% showed partial response (PR), 19.6% had stable disease (SD), and 1.1% had progressive disease (PD). pCR was achieved in 2.2% of patients, all of whom were in the GH group.
The primary endpoint, i.e., achieving the pre-established target tumor size for BCS, was reached in 69.6% (64/92, 95% CI: 59.1%–78.7%) of patients, significantly surpassing the set goal of 50.8% (P < 0.001). The rate was 73.5% in the GH and 58.3% in the GL group. The EOT surgical plan of BCS was 62.0% (57/92, 95% CI: 51.2%–71.9%; 67.6% for GH and 45.8% for GL). Finally, two of the 57 patients initially planned to undergo BCS eventually underwent total mastectomy due to positive resection margins. The actual overall BCS rate was 59.8% (55/92, 95% CI: 49.0%–69.9%; 64.7% for GH and 45.8% for GL) (Fig. 2). The overall response and choice of surgery were similar between premenopausal and postmenopausal patients (Fig. 3).
Bar graphs show (1) the rate of achieving the target tumor size for BCS based on imaging, (2) the actual BCS rate, and (3) the proportion of patients with a BCS surgical plan at the end of treatment. Results are shown for the total cohort (gray), genomic high-risk group (black), and genomic low-risk group (white). Error bars represent 95% confidence intervals.
Further, 142 adverse events were reported in 42 patients (35 patients with NCT [51.5%] and 7 patients with NET [29.2%]). Most of the reported adverse reactions were of grade 1 or 2, with no severe adverse events observed beyond expectations (Supplementary Table 1). None of the pre-treatment clinical or pathological factors significantly predicted BCS conversion (Supplementary Table 2).
Exploratory analysis according to four groups of the MammaPrint index
Exploratory analysis revealed that of the total 92 patients, 11 were categorized as H2 (12.0%), 57 as H1 (62.0%), 21 as LR (22.8%), and 3 as UL (3.2%). The H2 group showed a higher rate of achieving target size than the H1 group (90.9% vs. 70.2%), and the UL group showed a higher rate than the LR group (100% vs. 52.4%), although neither difference was significant (P > 0.05).
Discussion
This study evaluated the effectiveness of pre-treatment multigene assays in guiding NCT or NET to achieve improved BCS rates. The primary endpoint of achieving the pre-established target tumor size for BCS was reached in 69.6% of patients with ER+/HER2– breast cancer initially deemed unsuitable for BCS.
The PLATO study stands out from previous research in several key aspects. Notably, we engaged an independent panel of experienced surgeons to assess BCS feasibility and study eligibility, ensuring an unbiased evaluation process. Additionally, the requirement for pre-treatment determination of a target tumor size for BCS, primarily based on MRI, established a clear, objective benchmark for treatment efficacy. Furthermore, the flexibility allowed the extension of the period of NET beyond the standard 16 weeks to a maximum of 24 weeks, which introduced a tailored approach to patient care. Importantly, our study included a large proportion of premenopausal patients (64.1%), who generally exhibit a stronger preference for breast preservation.
Similar to the PLATO study, Bear et al. performed a pilot study of 64 patients using the 21-gene assay (Oncotype DX) for guiding NCT or NET to facilitate BCS13, with the primary endpoint not being BCS rate or BCS conversion rate but rather refusal rate of assigned treatment in the randomized patients. They reported a BCS conversion rate of 72%–75% with NET in patients with low or intermediate RS and 57%–64% with NCT in patients with high or intermediate RS. The overall BCS conversion rate was similar to that in our study, although our study showed a higher rate of BCS conversion in NCT than in NET.
A critical limitation of this type of study on BCS conversion lies in the objective determination of BCS eligibility. In most studies, BCS eligibility was evaluated by operating surgeons13,14. To increase the objectivity of the judgment of BCS eligibility, we used the two unique processes described above: (1) a panel of three independent judges and (2) pre-recorded target tumor size for each patient. The tumor size is the most significant factor for the choice of total mastectomy versus BCS. A systematic review investigating factors influencing the choice of surgery found that rates of mastectomy increased with larger tumor size15. However, no absolute size threshold has been established. The type of surgery depends on factors such as tumor ___location in the breast, distance from the nipple, patient’s breast size, breast redundancy, patient’s age, and the operating surgeon’s preference16.
The utilization of the 70-gene assay (MammaPrint) for genomic risk classification yielded a higher proportion of high-risk patients than anticipated, contrasting with expectations based on the MINDACT trial outcomes (64.1% genomic low-risk)12. However, in our study, 73.9% of patients were classified as genomic high-risk, and the reason for this is uncertain. This might be due to the inclusion of clinically higher-risk patients in our study, with larger tumors and/or axillary lymph node involvement, because only patients needing neoadjuvant therapy and total mastectomy candidates could be included. Another potential factor contributing to the higher-risk classification could be the use of core biopsy specimens available before neoadjuvant therapy rather than the use of surgical specimens. In a study that used a 70-gene assay for core biopsy specimens of patients receiving neoadjuvant chemotherapy, 86% were classified as genomic high-risk17. In a study analyzing the National Cancer Database of the USA of patients who received neoadjuvant chemotherapy, 84.6% of patients were high-risk with the 70-gene assay, while 57.7% were high-risk with the 21-gene assay (Oncotype DX)18. In the study by Bear et al., which also used core biopsy specimens for the 21-gene assay to choose neoadjuvant therapy, only 23.7% of patients were high-risk13. Furthermore, Audeh et al. conducted a 70-gene assay of patients in the Neoadjuvant Breast Symphony Trial (NBRST) and showed that 76.8% of patients were classified as high-risk19. The high proportion of high-risk results can be a disadvantage for using the 70-gene assay for individualized strategy selection of NCT vs. NET, given that more patients have to receive chemotherapy.
This study included a relatively high proportion of premenopausal women (64.1%) and showed that this strategy could be helpful for young women with a strong desire for breast conservation. The overall response and rate of achieving target size were similar between premenopausal and postmenopausal women. However, concerns persist regarding the use of multigene assays in premenopausal women. In an exploratory analysis of updated results of the MINDACT trial, women aged ≤50 years with high-clinical/low-genomic risk (70-gene assay) had an absolute distant metastasis-free survival benefit of 5% at 8 years with the addition of adjuvant chemotherapy20. Furthermore, in the RxPONDER study for lymph node-positive patients, premenopausal women had significant chemotherapy benefits even with low 21-gene RS21. The American Society of Clinical Oncology guideline update for biomarkers published in 2022 recommended that clinicians should not use the MammaPrint test for patients aged ≤50 years, and the Oncotype DX test should not be offered to premenopausal node-positive patients22. Future clinical trials will shed light on whether ovarian function suppression would replace chemotherapy in these patients. In our study, we could observe endocrine therapy response in low-risk patients and recommend adjuvant chemotherapy in cases of disease progression during NET as a second safety check.
There are several disadvantages associated with implementing this strategy in clinical practice. We used eight cycles of preoperative anthracyclines and taxanes in our study, and 50% of the patients were found to be lymph node-negative at surgery. A significant proportion of these patients might have been true lymph node-negative before neoadjuvant therapies, as lymph node complete remission is uncommon in the ER+/HER2– population23. If we had treated these patients with surgery first rather than neoadjuvant therapy, the lymph node-negative patients would have received four cycles of adjuvant chemotherapy rather than eight cycles. Moreover, 11.9% of patients in our study had pN2 or N3 disease, which is not an indication of using genomic assays according to current guidelines22,24.
We did not find any clinical or presurgical pathological factors significantly associated with BCS conversion. Although not statistically significant due to the small sample size, there were numerical differences according to the four-level classification (subcategories) of the MammaPrint index. Among genomic high-risk patients receiving NCT, target size achievement rate and actual BCS rate were higher in H2 patients than in H1 patients, and among genomic low-risk patients receiving NET, UL showed higher BCS conversion than LR. Consistent with our study findings, a previous study showed a significantly higher percentage of pCR in H2 tumors (23%) than in H1 tumors (6.1%) in NCT-treated patients in the NBRST25. To the best of our knowledge, our study is the first to show the possibility of a better response to NET in UL patients than in LR patients. The target size achievement rate was 100.0% in UL compared with the 52.4% in LR.
This study has some limitations. First, we were unable to recruit the preplanned number of patients due to a delay in patient enrollment. Second, our study was not randomized and lacked a control arm; we used historical data as a control. This study included only Asian women with relatively small-sized breasts. The general breast conservation rate for early breast cancer in Korea was 68.6% in 201926. The preference for breast conservation is different across countries. In the CALGB 40603 study, only 68% of women who converted from BCS-ineligible to BCS-eligible with neoadjuvant therapy chose breast conservation14. In contrast, 86.9% of BCS-converted patients with NCT chose BCS in a Korean study27. In the BrighTNess study, 79.6% of BCS-eligible European and Asian patients chose BCS after neoadjuvant therapy in contrast to 55.0% of North American patients28. Our strategy might not be highly applicable in North America and other countries where the BCS rate is low.
In conclusion, for women with ER+/HER2– breast cancer seeking breast preservation but facing challenges with borderline or impossible BCS mainly due to tumor size, our study recommends pre-treatment multigene assays to guide the choice between NCT and NET. This approach significantly increases the chances of achieving BCS while avoiding unnecessary chemotherapy in patients where it is not needed. Our study highlights the feasibility of this strategy in clinical practice.
Methods
Study design and patients
This PLATO study (NCT03900637) was a multicentre, phase II, prospective cohort study conducted from April 2019 to December 2023 across four large tertiary hospitals in South Korea (Seoul National University Hospital, Asan Medical Center, Seoul National University Bundang Hospital, and Korea Cancer Center Hospital). All participating centers received approval from their institutional review boards for this study. All patients provided written informed consent.
The main inclusion criteria were clinical stage II-IIIA, ER+/HER2– breast cancer with measurable tumor size, and BCS unfeasible considering the tumor size, tumor ___location, and breast size. The exclusion criteria were diffuse malignant microcalcification, multicentric breast cancer (multiple tumors in different quadrants), bilateral breast cancer, distant metastasis, history of breast treatment, history of other cancer, and male patients. Operating surgeons primarily decided on BCS feasibility in each patient before inclusion in this study. Subsequently, imaging files (magnetic resonance imaging [MRI], mammography, and ultrasonography) and physical examination findings, if available, were independently reviewed by a panel of two independent experienced surgeons not involved in patient recruitment. The panel judged BCS feasibility and study eligibility. In cases of discordance between the two surgeons’ opinions, the images were evaluated by a third surgeon for the final decision. A total of 130 patients were initially screened, with 100 enrolled and 92 finally analyzed (Fig. 1). Before therapy initiation, each surgeon recorded a target tumor size at which the surgeon could conduct BCS, considering the tumor ___location and breast size. This decision was predominantly based on MRI tumor size (n = 86, 93.5%), and in a smaller proportion with ultrasonography size (n = 6, 6.5%).
Multigene assay and treatment allocation
All patients underwent testing with the 70-gene assay (MammaPrint) using core needle biopsy specimens before neoadjuvant therapy initiation. Ten unstained slides of formalin-fixed paraffin-embedded tumor tissue were prepared for the 70-gene assay and sent to Agendia Inc. Patients were assigned to treatment based on the results of the 70-gene assay. Those classified as low-risk by the 70-gene assay received NET, while those classified as high-risk received NCT. The MammaPrint index was used to further categorize patients as UltraLow (UL; +1.000 to +0.356), Low-Risk (LR; +0.355 to +0.001), High 1 (H1; 0.000 to –0.569), and High 2 (H2; –0.570 to –1.000).
The NCT regimen included four standard cycles of anthracycline + cyclophosphamide (AC) every 3 weeks, followed by four cycles of docetaxel every 3 weeks or 12 cycles of paclitaxel weekly. In the NET regimen, postmenopausal women received letrozole (2.5 mg per day) for 16 weeks. Premenopausal women received leuprorelin (3.75 mg subcutaneously) every 4 weeks with letrozole for 16 weeks. The duration of NET could be extended to a maximum of 24 weeks based on the physician’s decision.
Evaluation of BCS conversion and actual BCS rate after neoadjuvant therapy completion
BCS eligibility after NET or NCT was determined based on whether the residual tumor size on imaging after neoadjuvant therapies was equal to or smaller than the pre-established target size recorded by the surgeon. The choice between BCS and total mastectomy was made after discussions with patients, occasionally involving multidisciplinary team discussions. In cases where the resection margin was positive for tumor cells after BCS, the decision of re-excision or total mastectomy was made by the operating surgeon.
Adjuvant therapy and follow-up
After surgery, each patient received adjuvant therapy according to the guidelines of the respective trial centers. Adjuvant endocrine therapy was recommended for all patients, whereas adjuvant chemotherapy was recommended for patients with disease progression during NET. For exploratory analysis, disease recurrence or survival would be monitored in post-surgery patients for a follow-up period of 5 years, according to institutional follow-up policy.
Study endpoints
The primary endpoint was achieving a rate of conversion, from BCS-ineligible to BCS-eligible, of more than 50.8%. The secondary endpoints included actual overall BCS rate, pCR rate, and clinical response rate. A previous study reported the conversion rate from BCS-ineligible to BCS-eligible with NCT of 35.8% in Korean patients with HR+/HER2– breast cancer27.
Sample size calculations
We assumed that with our study regimen, the BCS conversion rate would be increased to 50.8% (15% increase). Given these estimates, with a 10% type II error rate and 90% power, the target enrollment was set at 122 patients. However, due to delays in patient enrolment, accrual was closed at 100 participants in December 2023.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR), and categorical variables as frequency and percentage. Continuous variables were compared between groups using the Wilcoxon rank sum test, and categorical variables using Pearson’s χ2 test or Fisher’s exact test. A P-value < 0.205 was considered statistically significant.
The rate of achieving target size and actual BCS rate were calculated with two-sided binomial confidence intervals (CIs) of 95% for the high-risk, low-risk, and overall groups. Differences between groups were compared using Pearson’s χ2 test or Fisher’s exact test. All statistical analyses were performed using R 4.3.0.
Data availability
Deidentified participant data will be made available upon publication of the study. Researchers who wish to access the data must submit a Research Collaboration Proposal Request Form to Wonshik Han ([email protected]) or Byung Ho Son ([email protected]). Access will be granted to researchers who provide a methodologically sound proposal that aligns with the aims outlined in the approved submission. The data will be available for specified purposes only, and access will be provided upon the signing of a formal data access agreement.
References
Fisher, B. et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 16, 2672–2685 (1998).
van der Hage, J. A. et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J. Clin. Oncol. 19, 4224–4237 (2001).
Wolmark, N., Wang, J., Mamounas, E., Bryant, J. & Fisher, B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 30, 96–102 (2001).
Mieog, J. S., van der Hage, J. A. & van de Velde, C. J. Neoadjuvant chemotherapy for operable breast cancer. Br. J. Surg. 94, 1189–1200 (2007).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Eiermann, W. et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann. Oncol. 12, 1527–1532 (2001).
Smith, I. E. et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate preoperative anastrozole, tamoxifen, or Combined with tamoxifen (Impact) multicenter double-blind randomized trial. J. Clin. Oncol. 23, 5108–5116 (2005).
Masuda, N. et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 13, 345–352 (2012).
Pease, A. M., Riba, L. A., Gruner, R. A., Tung, N. M. & James, T. A. Oncotype DX® recurrence score as a predictor of response to neoadjuvant chemotherapy. Ann. Surg. Oncol. 26, 366–371 (2019).
Akashi-Tanaka, S. et al. 21-Gene expression profile assay on core needle biopsies predicts responses to neoadjuvant endocrine therapy in breast cancer patients. Breast 18, 171–174 (2009).
Iwata, H. et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res. Treat. 173, 123–133 (2019).
Cardoso, F. et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 375, 717–729 (2016).
Bear, H. D. et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J. Surg. Oncol. 115, 917–923 (2017).
Golshan, M. et al. Impact of neoadjuvant chemotherapy in stage II–III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann. Surg. 262, 434–439 (2015).
Gu, J. et al. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin. Breast Cancer 18, e539–e554 (2018).
Han, Y. et al. The percentage of unnecessary mastectomy due to false size prediction using preoperative ultrasonography and MRI in breast cancer patients who underwent neoadjuvant chemotherapy: a prospective cohort study. Int. J. Surg. 109, 3993–3999 (2023).
Straver, M. E. et al. The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 119, 551–558 (2010).
Freeman, J. Q. et al. Evaluation of multigene assays as predictors for response to neoadjuvant chemotherapy in early-stage breast cancer patients. npj Breast Cancer 9, 33 (2023).
Audeh, W., Ramaswamy, H., Menicucci, A. & FLEX Investigators’ Group. Prediction of Chemotherapy Benefit by MammaPrint® in Patients with HR+HER2- Early-Stage Breast Cancer from Real-World Evidence Studies. 41st Annual Miami Breast Cancer Conference® - Abstracts. 38, 29 (2024).
van Piccart, M. et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 22, 476–488 (2021).
Kalinsky, K. et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N. Engl. J. Med. 385, 2336–2347 (2021).
Andre, F. et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J. Clin. Oncol. 40, 1816–1837 (2022).
Kim, H. J. et al. Efficacy of neoadjuvant endocrine therapy compared with neoadjuvant chemotherapy in pre-menopausal patients with oestrogen receptor-positive and HER2-negative, lymph node-positive breast cancer. Breast Cancer Res. 22, 54 (2020).
NCCN Clinical Practice Guideline in Oncology: Breast Cancer. ver. 2 (National Comprehensive Cancer Network, 2024).
Beitsch, P. D. et al. MammaPrint Index as a predictive biomarker for neoadjuvant chemotherapy response and outcome in patients with HR+HER2- breast cancer in NBRST. J. Clin. Oncol. 41, 521 (2023).
Choi, J. E. et al. Breast cancer statistics in Korea, 2019. J. Breast Cancer 26, 207–220 (2023).
Mo, H. et al. Actual conversion rate from total mastectomy to breast conservation after neoadjuvant chemotherapy for stages II–III breast cancer patients. J. Breast Dis. 5, 51–56 (2017).
Golshan, M. et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from the BrighTNess randomized clinical trial. JAMA Surg. 155, e195410 (2020).
Acknowledgements
This research received financial support and drugs from the following companies: Takeda Pharmaceutical Co., Ltd., Kwang Dong Pharmaceutical Co., Ltd., Shin Poong Pharmaceutical Co., Ltd., HyupJin Corporation, and Agendia, Inc.
Author information
Authors and Affiliations
Contributions
Dr. Han W. had full access to all of the data in the study and took responsibility for the integrity and accuracy of the data analysis. Concept and design: Han W., Jung JGAcquisition, analysis, or interpretation of data: Han W., Kang E., Jung J.G., Kim H.K., Lee H.B., Kim J., Shin H.C., Kim H.A., Kim E.K., and Son BHDrafting of the manuscript: Han W., Kang ECritical review of the manuscript for important intellectual content: Han W., Son B.H., Kim E.K., Kim HAStatistical analysis: Kang E., The Medical Research Collaborating Center (MRCC) of Seoul National University Hospital Biomedical Research Institute Obtained funding: Han WAdministrative, technical, or material support: Kang E., Jung J.G., Kim H.K., Lee H.B., Kim J., LEE S.B., Park C.S., Seong MKSupervision: Son B.H., Kim E.K., Kim H.A.
Corresponding author
Ethics declarations
Competing interests
H.W. and L.H.B. are co-founders and members of the DCGen Co., Ltd board of directors. L.H.B. received research funding from Devicor Medical Product, Inc., and consulting fees from Need Inc., outside the current work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, W., Kang, E., Jung, J.G. et al. Personalized neoadjuvant strategy using 70-gene assay to increase breast-conserving surgery in ER+/HER2– breast cancer. npj Breast Cancer 11, 57 (2025). https://doi.org/10.1038/s41523-025-00772-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-025-00772-5