Abstract
Glymphatic alterations may underlie neurodegeneration in alpha-synucleinopathies. Reduced Diffusion-Tensor Imaging ALong the Perivascular Space (DTI-ALPS), a proxy of perivascular glymphatic activity, has been scarcely studied in isolated REM sleep behaviour disorder (iRBD), a prodromal synucleinopathy stage. Furthermore, its associations with clinical symptoms and brain structural abnormalities remain unexplored. We assessed the DTI-ALPS in sixty-two patients with iRBD and twenty-three healthy controls (HC), exploring its associations with clinical symptoms, cortical thickness and brain volumetric data. iRBD patients exhibited a lower DTI-ALPS and poorer odor identification, semantic fluency and processing speed relative to HC. The DTI-ALPS positively correlated with cognitive performance, olfactory function and amygdalar, hippocampal, brainstem and diencephalic volumes, and negatively with age in iRBD. Perivascular glymphatic activity is compromised in iRBD and is associated with brain atrophy and clinical risk factors of progression to alpha-synucleinopathies, supporting the potential of the DTI-ALPS index as an early imaging neurodegeneration marker.
Similar content being viewed by others
Introduction
Alpha-synucleinopathies are a group of neurodegenerative diseases characterized by the aggregation of misfolded alpha-synuclein causing neuronal dysfunction and cell death1,2. Intra- and inter-neuronal alpha-synuclein accumulation – constituting Lewy body pathology- underlies Lewy Body disorders (LBD), namely Parkinson’s disease (PD) and dementia with Lewy Bodies (DLB), whereas oligodendroglial cytoplasmic aggregates are a neuropathological feature of multiple system atrophy (MSA). Among the different factors proposed to contribute to synuclein deposition, alterations in brain metabolic clearance driven by the glymphatic system have been suggested3,4,5,6.
The glymphatic system is a complex cerebrospinal fluid (CSF) transport system involved in brain waste removal. The activity of the glymphatic system is high during slow wave sleep, in which interstitial spaces widen and CSF clearance rate is increased, thereby allowing the flushing of toxins and proteins accumulated during wakefulness7,8. In this process, CSF flows from the peri-arterial spaces into the interstitial space, flushing solutes accumulated in the brain parenchyma to the peri-venous spaces of meningeal and cervical lymphatic vessels. CSF inflow across peri-arterial, interstitial and peri-venous spaces is mainly driven by cardiovascular pulsations9,10 and occurs through Aquaporin 4 (AQP4) water channels, expressed in the end-feet of astroglial cells in perivascular spaces7,11. Preclinical research in animal models has shown that experimental suppression of glymphatic activity impairs removal of amyloid-b7, tau and alpha synuclein12,13,14, fostering the formation of protein aggregates, well-established neuropathological features of Alzheimer’s Disease (AD) and LBD, respectively.
In humans, the glymphatic system was first studied using contrast-enhanced magnetic resonance imaging (MRI) following intrathecal or intravenous gadolinium administration15,16. Recently, non-invasive methods have been developed to assess in vivo human glymphatic functioning. In this vein, the Diffusion Tensor Image analysis ALong the Perivascular Space (DTI-ALPS), uses diffusion-weighted imaging obtained with MRI to characterize the relative CSF movement in the direction of perivascular medullary vessels, involved in the transport of solutes from and to the outside of brain parenchyma17. Whereas the DTI-ALPS index cannot be equated to whole-brain glymphatic functioning, it shows strong correlations with the classical glymphatic clearance function after intrathecal gadolinium administration18, constituting a useful proxy of local brain metabolic clearance19. In this line, prior studies have reported reduced DTI-ALPS measures in neurodegenerative diseases, such as AD17 and PD. In alpha-synucleinopathies, a decreased DTI-ALPS has been reported20,21,22,23,24,25,26,27, which has been hypothesized to reflect dysfunctional metabolic clearance, potentially enhancing synuclein deposition28 and contributing to disease progression5. Remarkably, the diagnosis of alpha-synucleinopathies is often preceded by a prodromal period characterised by non-motor symptoms that may arise up to 20 years before the onset of the main diagnostic clinical features29. Therefore, the study of DTI-ALPS indicators in prodromal stages is warranted to assert the presence of glymphatic disturbances in early stages of neurodegeneration.
Isolated Rapid-Eye-Movement (REM) sleep behavior disorder (iRBD) is a parasomnia characterised by the loss of muscle atonia in REM sleep, leading to vigorous dream-enacting behaviors30. Together with other non-motor symptoms, such as olfactory impairment and dysautonomia, iRBD has been established as a relevant prodromal symptom of alpha-synucleinopathies29, with patients with iRBD displaying a conversion risk exceeding 90% in a 14-year follow-up31. In this line, the presence of neuropsychological impairments and brain structural and functional changes is well-established in iRBD patients, which show similar alterations to those manifested in later stages of neurodegeneration32,33,34. Regarding glymphatic dysfunction, few studies have investigated DTI-ALPS in early synucleinopathies, reporting lower ALPS values in iRBD patients relative to healthy controls (HC)35,36,37. Altogether, these results suggest local alterations in physiological mechanisms relevant to glymphatic clearance in prodromal alpha-synucleinopathies. While the associations between DTI-ALPS and clinical features in iRBD remain unclear, these studies are limited by small sample sizes36,37 and by the inclusion of patients in whom RBD was suspected but not confirmed by video-polysomnography35. These studies lack comprehensive assessments, which precludes investigation of the associations between altered DTI-ALPS and its impact in iRBD. Furthermore, to our knowledge, no prior study has assessed the relationship between DTI-ALPS indices and other structural brain imaging neurodegeneration markers in patients with iRBD.
The objective of the present study is to investigate local brain glymphatic dysfunction by means of the DTI-ALPS index in a well-characterised sample of patients with video-polysomnography confirmed iRBD and its associations with clinical symptomatology, neuropsychological functioning and brain volumes in different regions. We hypothesize that patients with iRBD will show decreased DTI-ALPS, as a marker of impaired glymphatic clearance, and that this will be associated with brain atrophy and cognitive dysfunction.
Results
Sociodemographic and clinical analyses
The final sample consisted of 62 patients with iRBD and 23 HC.
The sociodemographic and clinical characteristics of the participants are summarized in Table 1. iRBD and HC groups were similar in age, sex, education years and estimated premorbid intelligence as assessed with the Vocabulary subtest of the WAIS-IV. There were significant intergroup differences in general cognitive status and questionnaires assessing non-motor and neuropsychiatric symptoms. Patients with iRBD scored lower in global cognition and showed significantly more depressive symptoms, apathy, non-motor and neuropsychiatric symptoms compared to HC.
Intergroup comparisons in DTI-ALPS
Regarding the DTI-ALPS index, patients with iRBD showed significantly lower values of bilateral DTI-ALPS index relative to HC (MHC = 1.53, SDHC = 0.12; MiRBD = 1.45, SDiRBD = 0.16; t = 2.527, p = 0.014, Cohen’s d = 0.531, see Fig. 1). No significant intergroup differences were found in the three control ROIs placed along the corpus callosum (tROI1 = -0.606, p = 0.282; tROI2 = -0.161, p = 0.439; tROI3 = 0.192, p = 0.143).
Left Group differences in bilateral DTI-ALPS index. Right Significant correlations between bilateral DTI-ALPS index and age, UPSIT-40 scores, SDMT and semantic fluency. Data corresponding to iRBD are presented in red, whereas data for HC are presented in blue. Abbreviations: DTI-ALPS, diffusion tensor imaging along the perivascular space; HC, healthy controls; iRBD, isolated Rapid-Eye-Movement disorder; r, correlation coefficient of the iRBD group; SDMT, Symbol-Digit Modality Test- oral version; p, p-value for correlations in the iRBD group; UPSIT-40, University of Pennsylvania Smell Identification Test, 40 items. Graphs were obtained using the package ggplot2 (Wickham, 2016).
Neuropsychological performance
Neuropsychological data for iRBD patients and HC are presented in Table 2.
Analyses of neuropsychological data revealed significant intergroup differences. Patients with iRBD exhibited lower scores in the digits span forward, JLO, SDMT, Stroop Word test, semantic fluency, GPT with the non-dominant hand and UPSIT-40. Only intergroup differences in UPSIT-40 survived FDR-correction for multiple comparisons.
Associations between the DTI-ALPS index, clinical features, and cognitive functioning
Associations between the DTI-ALPS index and significant clinical and neuropsychological variables were assessed for iRBD and HC and are presented in Fig. 1.
The DTI-ALPS index significantly correlated with performance in neuropsychological tests. Specifically, significant positive correlations were found between the DTI-ALPS index and SDMT (r = 0.277, p = 0.032), semantic fluency (r = 0.305, p = 0.018) and UPSIT-40 scores (r = 0.386, p = 0.002). No significant correlations between the DTI-ALPS index and performance in neuropsychological tests were found in HC group.
The association with age in both groups was also tested. A significant negative correlation between DTI-ALPS index and age was found only in the iRBD group, showing that a higher age is associated with a decreased DTI-ALPS index (r = -0.295, p = 0.020).
Only the correlation between DTI-ALPS and UPSIT-40 in the iRBD group remained significant after FDR-correction for multiple comparisons and after including age in the regression model (see Supplementary Figure 1, showing correlation between UPSIT-40 and bilateral DTI-ALPS age-corrected residuals).
Atrophy measures
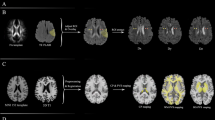
Regarding intergroup volumetric differences in subcortical and global GM measures, significant group effects were observed. Patients with iRBD showed significantly lower volumes in bilateral amygdala (t = 33.858, p = 0.006), hippocampus (t = 34.243, p = 0.006), ventral diencephalon (t = 31.579, p = 0.007) and brainstem (t = 22.439, p = 0.030). Lower volumes in global brain measures were observed in patients with iRBD, with decreased cortical (t = 30.681, p = 0.003) and subcortical volumes (t = 25.619, p = 0.0138) as well as decreased total GM volume (t = 32.014, p = 0.005). Vertex-wise cortical thickness analyses revealed non-significant differences between groups. No significant intergroup differences were found in WM and non-WM hypointensities (U = 546, p = 0.199; U = 720, p = 0.841).
Associations between the DTI-ALPS index and volumetric data
Significant associations were found between the DTI-ALPS and volume of structures showing atrophy in the iRBD group (Fig. 2). Positive age-corrected correlations were found between the DTI-ALPS and amygdalar (riRBD=0.564, p =< 0.001), hippocampal (riRBD=0.562, p =< 0.001), ventral diencephalon (riRBD=0.559, p =< 0.001) and brainstem (riRBD=0.503, p =< 0.001) volumes. No significant associations were found between the DTI-ALPS and subcortical volumetric data in the HC group and no age-group interaction effects were found.
Correlation plots showing the relationship between the residuals of the DTI-ALPS and volumetric data after adjusting for age in the regression model. Data corresponding to iRBD are presented in red, whereas data for HC are presented in blue. DTI-ALPS diffusion tensor imaging along the perivascular space; B, correlation coefficient for the iRBD group; p, p-value for the correlations of the iRBD group. Graphs were obtained using the package ggplot2 (Wickham, 2016).
Regarding global atrophy measures, significant positive age-corrected correlations were observed in the iRBD and HC groups between the DTI-ALPS and total GM volume (riRBD=0.721, p =<0.001; rHC = 0.571, p = 0.006) and cortex volume (riRBD=0.689, p =<0.001; rHC = 0.532, p = 0.011).
Discussion
The present study unveils the associations between the DTI-ALPS, a local measure of glymphatic activity, and clinical symptoms and abnormal brain morphology in patients with iRBD. Particularly, a reduced DTI-ALPS was associated with olfactory impairments, and deficits in processing speed and semantic fluency in iRBD patients. Furthermore, low DTI-ALPS was associated with atrophy in subcortical structures, such as the amygdala, hippocampus, ventral diencephalon and brainstem in patients with iRBD. To our knowledge, this is the first study to assess glymphatic functioning in relation to neuropsychological performance and brain volume in patients with iRBD. Our findings suggest that there is a perivascular glymphatic dysfunction in iRBD which is associated with clinical and neuropsychological risk factors of progression to overt alpha-synucleinopathies, and brain atrophy.
In the present study, we found a reduced DTI-ALPS index in patients with iRBD relative to age-matched HC, building on results from prior studies with smaller sample sizes36,37 and non-vPSG-confirmed iRBD patients35. However, the contribution of a reduced DTI-ALPS to clinical measures in prior iRBD studies remained unclear. Whereas Lee et al. and Bae et al. did not find any association between the ALPS-index and clinical variables, Si et al. reported a negative correlation with RBD severity in patients with probable RBD35. Altogether, these findings suggest that impaired local glymphatic clearance along perivascular spaces occurs already in early stages of alpha-synucleinopathies.
Reports of a decreased ALPS in iRBD align with similar findings in PD21,22,24,25,27,28,38,39, in which a low ALPS has been associated with motor symptom severity24,25,27,39 and disease duration27. In PD, some studies fail to find any association between DTI-ALPS and cognitive functioning21,24,25,39, while others report positive relationships between this index and scores in cognitive screening tests20,22,38,40. Importantly, only few ALPS studies in PD22,38, and none in iRBD, include a comprehensive assessment of neuropsychological functioning and other non-motor features, such as olfactory dysfunction, which are affected early in the neurodegenerative process29,41. For example, the study by Pang et al. showed that a reduced ALPS in PD with mild cognitive impairment (PD-MCI) was associated with lower performance in attentional tasks (digit span and TMT)38. Potential links between glymphatic dysfunction and cognitive deficits in prodromal and established alpha-synucleinopathies may be unveiled with the incorporation of extensive neuropsychological examination in glymphatic studies, according to current research recommendations42,43.
To our knowledge, this is the first study to show an association between the ALPS-index and neuropsychological performance in iRBD patients. In our study, a reduced DTI-ALPS in iRBD was associated with low processing speed, as assessed with the oral version of the SDMT, and decreased semantic fluency. Reduced processing speed is considered the most prominent neuropsychological feature in iRBD33,44,45,46,47, strongly contributing to executive functioning deficits33, which are well-established markers of conversion into dementia forms of LBD48,49. Relevantly, slowness in information processing has been related with altered resting-state functional dysconnectivity in posterior cortical regions44. In this line, deficits in semantic verbal fluency have also been consistently reported in iRBD34,48,50, showing strong predictive value to detect conversion into dementia over time48,50. Therefore, our results suggest that decreased local glymphatic activity is associated with core features of the neuropsychological profile of iRBD patients.
A relevant finding of our study is that reduced perivascular glymphatic activity in patients with iRBD was linked to olfactory dysfunction, a well-established risk factor for conversion to alpha-synucleinopathies29,41,51. Interestingly, recent research has pointed to olfactory and peri-olfactory regions as key anatomical hubs in CSF outflow, suggesting a relevant role of this anatomical ___location in glymphatic clearance52,53. In fact, the most predominant CSF outflow pathway circulates alongside olfactory sensory nerves, transversing the cribriform plate to ultimately reach the nasal mucosa and cervical lymph nodes52. Based on this anatomical pathway, the existence of reciprocal influences between olfactory/nasal and CSF alterations have been proposed. On the one hand, besides its role in glymphatic clearance, CSF is known to provide a route for signal transmission within brain. Therefore, altered CSF flow could potentially be linked to neurological dysfunction, including olfactory impairments52. In the reverse direction, it has been proposed that olfactory epithelial damage could lead to a reduction in CSF outflow through the cribriform plate, thereby impairing glymphatic clearance52. The latter mechanism is of particular interest in early-alpha synucleinopathies, for which exposure to certain toxins, such as pesticides and solvents, that are known to penetrate the nervous system through the nasal mucosa, is a recognized risk factor for neurodegeneration54,55. To our knowledge, no prior evidence has been reported on the links between glymphatic dysfunction and olfactory impairments in iRBD nor in later stages of alpha-synucleinopathies, so further research is needed to clarify the mechanisms underlying this association.
Our findings are in line with prior research suggesting that a decreased ALPS in iRBD is associated with higher risk of conversion to alpha-synucleinopathies, although these findings are limited by a small sample size and did not survive corrections for multiple comparisons37. Similar results have been reported in PD, showing that the ALPS-index showed good discriminative power (AUC: 0.85) between PD converters to dementia from non-converters39. Altogether, these findings may suggest that local glymphatic alterations not only occur early in the neurodegenerative process in alpha-synucleinopathies but that they are associated with well-established risk factors of conversion to alpha-synucleinopathies and, potentially, to more severe phenotypes of the disease. Since iRBD patients are known to constitute a very heterogeneous group, future studies should address the potential differential involvement of the glymphatic system in iRBD subgroups stratified according to the presence of risk factors.
In our study, we have found decreased volume in subcortical structures in iRBD patients, affecting the amygdala, hippocampus, ventral diencephalon and the brainstem. Our results build on prior reports of reduced hippocampal volumes in iRBD45, along with orbitofrontal56 and temporo-occipital atrophy57. Remarkably, limbic regions and the brainstem are key anatomical hubs of the REM sleep circuit, and their disruption has been proposed to underlie core features of RBD, such as aggressive dream content and loss of physiological REM atonia57,58. Our results show that atrophy in these structures positively correlates with decreased DTI-ALPS only in iRBD patients, which suggests that glymphatic dysfunction could be associated with early volumetric changes seen in prodromal stages of alpha-synucleinopathies. However, our findings are exploratory and several aspects must be considered. First, both limbic and brainstem structures comprise small cellular nuclei anatomically and functionally differentiated which contribute to different REM sleep features. A more fine-grained segmentation of these structures would likely unveil atrophy in specific nuclei, rather than in the whole structure. Furthermore, we have not found cortical thickness reductions in iRBD, which suggests that cortical thinning, expected later in the disease, may not be evident in the studied iRBD sample. Since the ALPS-index focuses on microstructural aspects, potential associations could be studied with more sensitive measures of cortical microstructural damage, such as cortical mean diffusivity (cMD)59. Finally, we found that the DTI-ALPS positively correlated with global atrophy measures in both study groups. Although the structural correlates of the DTI-ALPS are not well-established yet, our findings are in line with those by Siow et al. (2022) who found a positive association between the DTI-ALPS index and GM volume in a healthy community sample60. Altogether, these findings could align with proposed links between glymphatic functioning, higher risk of pathological depositions and subsequent alterations in brain function and structure3,28. However, this remains a hypothesis and further longitudinal research is needed addressing the associations between the DTI-ALPS and other markers of glymphatic functioning with structural and functional brain features.
The investigation of the glymphatic system in early stages of alpha-synucleinopathies is of utmost relevance, since glymphatic failure has been proposed to contribute to alpha-synuclein deposition associated with disease progression4,61. Various factors and mechanisms have been proposed to contribute to this process, interacting in complex and intricate ways, and a vicious cycle between synuclein accumulation, glymphatic disfunction and neurodegeneration has been proposed. Fragmented sleep and altered sleep architecture3,4,62 due to motor events and severe dysautonomia63,64,65,66 associated with iRBD have been considered as a relevant factors contributing to glymphatic failure and subsequent synuclein pathology3,6. Severe dysautonomia in LBD also comprises altered cerebrovascular responses that may lead not only to decreased glymphatic synuclein clearance, but also to episodic hypoperfusion and hypoxia that may trigger acidification and neuroinflammatory responses, leading to further endothelial damage6. Finally, together with altered sleep architecture and dysautonomia, altered circadian rhythmicity in iRBD67,68,69,70,71,72,73 and overt alpha-synucleinopathies67,74,75,76,77 has been proposed as a contributing factor to glymphatic failure3, since glymphatic flow is known to display circadian rhythmicity78. Based on these models, future studies should investigate the relationships between glymphatic functioning, sleep architecture, cerebrovascular dysfunction, and circadian rhythms in iRBD.
The present study unveils the associations between altered glymphatic functioning and risk factors of conversion to synucleinopathies in patients with iRBD. However, several limitations must be considered. Our results are based on cross-sectional data, precluding to study the association between the ALPS-index and actual disease progression, which can only be addressed in longitudinal studies. Moreover, we did not assess the association between the DTI-ALPS and CSF biomarkers of neurodegeneration - i.e. synuclein, beta-amyloid, tau and phosphorylated-tau levels. Furthermore, we would like to emphasize that the DTI-ALPS is a measure of local diffusivity at a specific point of the glymphatic system19. Therefore, it does not capture the entire complexity of the brain clearance network, distributed across various anatomical regions.
Our study has several strengths. We have studied the DTI-ALPS index in a larger sample of vPSG-confirmed iRBD patients relative to prior studies. Moreover, we have used an automated method for ROI set-up in the standard space, which ensures further reproducibility relative to prior DTI-ALPS studies in iRBD, which perform manual ROI placement. Moreover, we have addressed the associations between DTI-ALPS index and non-motor symptoms as assessed in a comprehensive neuropsychological protocol. Further longitudinal studies are needed to address the associations between the DTI-ALPS, phenoconversion trajectories and neurodegeneration biomarkers to support its use as a disease progression marker in synucleinopathies.
Our study shows that patients with iRBD exhibit a low DTI-ALPS index, marker of local glymphatic activity in perivascular brain regions. Decreased DTI-ALPS in iRBD is associated with olfactory dysfunction, low processing speed and reduced semantic fluency, non-motor clinical risk factors for neurodegeneration. Moreover, perivascular glymphatic activity is related to brain atrophy measures. These findings underscore the potential usefulness of the DTI-ALPS as an imaging marker in prodromal stages of the synucleinopathies and highlight the importance of studying the contributions of glymphatic system alterations in early stages of neurodegeneration.
Methods
Participants
Consecutive participants were recruited from the Sleep Unit of the Neurology Service, at the Hospital Clínic de Barcelona (Catalonia, Spain). The total sample comprised 62 video-polysomnography-confirmed iRBD patients and 23 healthy controls (HC).
The inclusion criteria for iRBD patients were: (1) video-polysomnographic demonstration of REM sleep without atonia associated with dream-enacting behaviors, (2) absence of overt motor complaints at the time of the recruitment, (3) unremarkable neurological examination and (4) no temporal association between the estimated RBD onset and the introduction or withdrawal of a medication (Boeve, 2010; Iranzo et al., 2006).
The exclusion criteria for all participants were: (1) presence of dementia according to the Movement Disorders Society criteria79,80, (2) scores below 25 in the Mini-Mental State Examination81 (MMSE), (3) claustrophobia, (4) MRI artifacts and/or incidental pathological MRI findings, (5) conversion to an alpha-synucleinopathy during the time of the study, (6) presence of severe psychiatric or neurological comorbidities, (7) a scaled score below 7 in the Vocabulary subtest of the Wechsler Adult Intelligence Scale 4th edition (WAIS-IV), as an estimator of premorbid intellectual functioning, (8) specific exclusion criteria for the olfactory function assessment included history of nasal bone fracture, nasal polyps, diagnosis of rhinitis, and upper respiratory tract infections in the two weeks prior to testing. Additionally, the exclusion criteria for HC included the presence of self-reported RBD symptomology or other major sleep disturbances disclosed in a sleep interview.
The present study was approved by the Bioethics Committee of the University of Barcelona (IRB00003099) and was conducted in accordance with the basic principles of the Declaration of Helsinki, among other relevant regulations and guidelines. All participants signed informed written consent following full explanation of the procedures.
Demographic, clinical and neuropsychological assessments
Sociodemographic information was collected for all participants at the time of the study. iRBD patients’ clinical features were registered, including the date of iRBD diagnosis, the interval, in years, from iRBD diagnosis to the time of the study, and parkinsonian motor symptoms, quantified using part III of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III)82. Neuropsychiatric and synucleinopathy-related non-motor symptoms were also assessed through the Cummings Neuropsychiatric Inventory (NPI)83, Beck Depression Inventory 2nd edition (BDI-II)84, Starkstein’s Apathy Scale (AS)85 and, Non-Motor Symptoms Questionnaire (NMS)86. Global cognitive status was evaluated based on the Spanish version of the MMSE81.
All participants underwent a comprehensive neuropsychological assessment based on the MDS Task Force guidelines43. The neuropsychological protocol included [1] digits span – forward and backwards- (WAIS-IV) and Trail Making Test, part A to assess attention and working memory; [2] Rey’s Auditory Verbal Learning Test (RAVLT) - total and delayed recall score-, and Warrington Recognition Memory for Faces to assess auditory-verbal and visual memory; [3] semantic (animals) and phonemic (letter “P”) fluencies, the Stroop Word-Color test and Trail Making Test, part B87, to assess executive functioning; [4] the Benton’s Judgment of Line Orientation (BJLO), the Clock Copying Test and the short version of the Benton’s Facial Recognition Test (BFRT, to assess visuospatial and visuoperceptual (VS/VP) abilities; [5] the Symbol Digit Modalities Test (SDMT)-oral version and Stroop Word test to assess information processing speed; [6] the Boston Naming Test (BNT) and Similarities (WAIS-IV), to assess language. Manual dexterity with the dominant and non-dominant hand was assessed using the Grooved Pegboard Test. Additionally, color discrimination was assessed with the Farnsworth-Munsell 100-Hue test (FM-100). The University of Pennsylvania Smell Identification Test, 40 items, (UPSIT-40)88 was used to assess odor identification.
MRI Acquisition
MRI data were acquired with a 3.0 T scanner (MAGNETOM Prisma, Siemens, Germany), at the Centre de Diagnòstic per la Imatge of the Hospital Clínic de Barcelona (Catalonia, Spain). In this study, the scanning protocol included high-resolution 3- dimensional T1-weighted images acquired in the sagittal plane (TR = 2400 ms, TE = 2.22 ms, TI = 1000 ms, 208 slices, FOV = 256 mm; 1 mm isotropic voxel). Regarding diffusion data, two diffusion-weighted images (DWI) with opposite phase-encoding direction (anterior-posterior and posterior-anterior) were acquired. A multi-shell sequence was utilized in both DWI acquisitions (TR = 3230 ms, TE = 89.20 ms, FoV = 210 mm, flip angle 78°, slice thickness = 1.5 mm and number of slices = 92). Each acquisition comprised 99 directions at b = 0, 1500 and 3000 s/mm2. For this analysis, diffusion weighted images were filtered to include 14 non-diffusion-weighted volumes and 94 volumes at b-value of 1500 s/mm2.
Diffusion MRI processing
Diffusion MRI was processed with FMRIB’S Diffusion Toolbox (FDT) from FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT), estimating diffusion metrics with a diffusion tensor imaging (DTI) approach. The processing started with the estimation and correction of susceptibility induced field distortions with top-up tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/topup). Next, Brain Extraction Tool (BET) was used to perform skull extraction from the image (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET), and eddy currents and movement in data was corrected using eddy tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/eddy). Fractional anisotropy (FA) maps and tensor components were obtained using a diffusion model fit (DTIFIT) (https://users.fmrib.ox.ac.uk/~behrens/fdt_docs/fdt_dtifit.html) to fit the DTI model in each voxel.
DTI-ALPS calculation
DTI-ALPS index was calculated separately for each brain hemisphere to evaluate both hemispheres separately and bilaterally. The index was computed in an automatic approach with all tensors in standard space. Two spherical ROIs of r = 4 mm were placed in association and projection fibres of each hemisphere in the Montreal Neurological Institute (MNI) template. MNI coordinates of the centres of the left and right ROIs in association fibres were (−38, −12, 26) and (38, −12, 26), respectively, and (−26, −12, 26) and (25, −12, 26) in projection fibres. ROI placement was based on the study by Zhang et al. (2021)18 and adjusted following visual inspection to ensure adequate ROI ___location on projection and association fibres in the FSL HCP1065 template.
Registration from subject tensor components to MNI space was achieved obtaining the affine, rigid and non-linear registrations from subject FA to FA template using Advanced Normalization Tools (ANTs) (https://stnava.github.io/ANTs/). Transformations obtained were applied to tensor components while retaining their original orientation to preserve the original diffusivity values. HCP-1065 1 mm FA map was used as template (https://git.fmrib.ox.ac.uk/fsl/data_standard/-/blob/FSL6-0-1/FSL_HCP1065_FA_1mm.nii.gz, https://brain.labsolver.org/hcp_template.html)89.
For each hemisphere, the DTI-ALPS index was calculated as the ratio of the diffusion along perivascular spaces (x-axis) with the diffusion along the other perpendicular direction of the dominant fibres (y for projections fibres and z for association fibres) (See Eq.1 and Fig. 3). A bilateral index was obtained as the average between the ALPS-index obtained in right and left hemispheres (Eq.2).
To control for potential bias in the DTI-ALPS due to the presence of crossing fibres, diffusivities along the x-axis were calculated in three ROIs placed along the corpus callosum, as a control region (see Supplementary Fig. 1).
Cortical Thickness and subcortical volumetric analyses
The estimation of cortical thickness (CTh) and subcortical segmentation were performed using the automated FreeSurfer stream version 7.1 available at https://surfer.nmr.mgh.harvard.edu/. The procedures in FreeSurfer included removal of nonbrain data, registration to Talairach space, intensity normalization, tessellation of the gray matter (GM) and white matter (WM) boundaries, automated topology correction, and accurate surface deformation following intensity gradients to identify tissue borders. CTh was calculated as the distance between the WM and GM surfaces at each vertex of the reconstructed cortical mantle(https://freesurfer.net/fswiki/FreeSurferMethodsCitation). Results for each subject were visually inspected, and the befitting manual corrections were also performed to ensure the accuracy of registration, skull stripping, segmentation, and cortical surface reconstruction (https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TroubleshootingData).
Automated subcortical segmentation was performed with FreeSurfer (version 7.1). Total Intracranial Volume (TIV) was obtained and used to correct volumetric data for inter-individual differences in head sizes ((volume/eTIV)*100). TIV-corrected global atrophy measures including total cortical GM, total subcortical GM and global cerebral WM were calculated. Bilateral volumetric data of subcortical structures (thalamus, caudate, putamen, pallidum, hippocampus, amygdala, and ventral diencephalon) were computed by averaging eTIV-corrected unilateral volumes. To assess the potential influence of vascular damage on the DTI-ALPS index, we obtained WM and non-WM hypointensities for each participant.
Statistical analyses
All analyses of sociodemographic, neuropsychological, and clinical data were performed with R Statistical Software (v4.3.1, R Core Team 2023). Intergroup differences in categorical variables were analysed through Pearson’s chi-squared test. Differences between groups in continuous variables were tested using Student’s t and Mann-Whitney’s tests depending on the variables meeting normality assumptions.
Intergroup differences in DTI-ALPS index and volumetric data were tested using Student’s T tests, applying false discovery rate (FDR) for multiple corrections. Intergroup comparisons in CTh were assessed using a vertex-by-vertex general linear model (FreeSurfer 7.1) and the Monte Carlo Simulation with 10,000 iterations was used to provide clusterwise correction for multiple comparisons; the cluster-defining threshold was set at 1.3, in both directions (abs).
Analyses of covariance were used to assess between-group differences in neuropsychological variables using the lm() function, with group (HC or iRBD) as a between-subjects factor and sex and years of education as covariates. Bivariate and age-corrected Pearson’s and Spearman’s correlations, depending on normality of the variables, were performed to assess the associations between the DTI-ALPS index and demographic, neuropsychological and other imaging data. In the iRBD group, correlations were used to study the relationship between the DTI-ALPS index and clinical data. The statistical significance threshold was set at p < 0.05 and effect sizes were computed as Cohen’s ds.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The underlying code for this study is not publicly available but may be made available to qualified researchers on reasonable request from the corresponding author.
References
Stefanis, L. et al. How is alpha-synuclein cleared from the cell? J. Neurochem. 150, 577–590 (2019).
Power, J. H. T., Barnes, O. L. & Chegini, F. Lewy bodies and the mechanisms of neuronal cell death in parkinson’s disease and dementia with lewy bodies. Brain Pathol. 27, 3–12 (2016).
Buongiorno, M. et al. Altered sleep and neurovascular dysfunction in alpha-synucleinopathies: the perfect storm for glymphatic failure. Front. Aging Neurosci. 15, 1251755 (2023).
Sundaram, S. et al. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neurosci. Biobehav. Rev. 103, 305–315 (2019).
Nedergaard, M. & Goldman, S. A. Glymphatic failure as a final common pathway to dementia. Science 370, 50–56 (2020).
Ryman, S. G. et al. Abnormal cerebrovascular activity, perfusion, and glymphatic clearance in lewy body diseases. Mov. Disord. 39, 1258–1268 (2024).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111–147ra111 (2012).
Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The glymphatic system: a beginner’s guide. Neurochem Res. 40, 2583–2599 (2015).
Kiviniemi, V. et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms?. J. Cereb. Blood Flow. Metab. J. Int Soc. Cereb. Blood Flow. Metab. 36, 1033–1045 (2016).
Mestre, H. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 9, 4878 (2018).
Iliff, J. J. & Nedergaard, M. Is there a cerebral lymphatic system?. Stroke J. Cereb. Circ. 44, S93–S95 (2013).
Zhang, Y. et al. Interaction Between the Glymphatic System and α-Synuclein in Parkinson’s Disease. Mol. Neurobiol. 60, 2209–2222 (2023).
Cui, H. et al. Decreased AQP4 expression aggravates ɑ-synuclein pathology in Parkinson’s disease mice, possibly via impaired glymphatic clearance. J. Mol. Neurosci. 71, 2500–2513 (2021).
Lapshina, K. V. & Ekimova, I. V. Aquaporin-4 and Parkinson’s Disease. Int. J. Mol. Sci. 25, 1672 (2024).
Naganawa, S., Taoka, T., Ito, R. & Kawamura, M. The glymphatic system in humans: investigations with magnetic resonance imaging. Invest Radio. 59, 1 (2024).
Naganawa, S. & Taoka, T. The glymphatic system: a review of the challenges in visualizing its structure and function with MR imaging. Magn. Reson Med. Sci. 21, 182–194 (2020).
Taoka, T. et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J. Radio. 35, 172–178 (2017).
Zhang, W. et al. Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage 238, 118257 (2021).
Taoka, T. et al. Diffusion tensor image analysis along the perivascular space (DTI-ALPS): revisiting the meaning and significance of the method. Magn. Reson Med Sci. MRMS J. Jpn Soc. Magn. Reson Med. 23, 268–290 (2024).
Bae, Y. J. et al. Glymphatic function assessment in Parkinson’s disease using diffusion tensor image analysis along the perivascular space. Parkinsonism Relat. Disord. 114, 105767 (2023).
Ma, X. et al. Diffusion tensor imaging along the perivascular space index in different stages of Parkinson’s disease. Front Aging Neurosci. 13, 773951 (2021).
Chen, H. L. et al. Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (DTI-ALPS) in patients with Parkinson’s Disease. Oxid. Med. Cell Longev. 2021, 4034509 (2021).
Wang, X. et al. MRI index of glymphatic system mediates the influence of locus coeruleus on cognition in Parkinson’s disease. Parkinsonism Relat. Disord. 123, 106558 (2024).
Meng, J. C. et al. Correlation of glymphatic system abnormalities with Parkinson’s disease progression: a clinical study based on non-invasive fMRI. J. Neurol. 271, 457–471 (2024).
Qin, Y. et al. Neuroimaging uncovers distinct relationships of glymphatic dysfunction and motor symptoms in Parkinson’s disease. J. Neurol. 270, 2649–2658 (2023).
Shi, C. et al. Impaired glymphatic clearance in multiple system atrophy: A diffusion spectrum imaging study. Parkinsonism Relat. Disord. 123, 106950 (2024).
Shen, T. et al. Diffusion along perivascular spaces as marker for impairment of glymphatic system in Parkinson’s disease. NPJ Park Dis. 8, 174 (2022).
McKnight, C. D. et al. Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Parkinsonism Relat. Disord. 89, 98–104 (2021).
Fereshtehnejad, S. M. et al. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain 142, 2051–2067 (2019).
Iranzo, A., Santamaria, J. & Tolosa, E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 15, 405–419 (2016).
Galbiati, A., Verga, L., Giora, E., Zucconi, M. & Ferini-Strambi, L. The risk of neurodegeneration in REM sleep behavior disorder: A systematic review and meta-analysis of longitudinal studies. Sleep. Med. Rev. 43, 37–46 (2019).
Fiamingo, G., et al. Neuropsychological evaluation of phenoconversion risk in REM sleep behaviour disorder: A scoping review. J. Sleep. Res. 32, e13873 (2023).
Leitner, C. et al. Neuropsychological changes in isolated REM sleep behavior disorder: a systematic review and meta ‑ analysis of cross ‑ sectional and longitudinal studies. Neuropsychol. Rev. 34, 1 (2024).
Mombelli, S. et al. A data- driven approach to neuropsychological features in isolated REM behaviour disorder: A latent class analysis. J. Neuropsychol. 17, 161–179 (2023).
Si, X. et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson’s disease. Npj Park Dis. 8, 1–9 (2022).
Lee, D. A., Lee, H. J. & Park, K. M. Glymphatic dysfunction in isolated REM sleep behavior disorder. Acta Neurol. Scand. 145, 464–470 (2022).
Bae, Y. J. et al. Altered brain glymphatic flow at diffusion-tensor MRI in rapid eye movement sleep behavior disorder. Radiology 307, e221848 (2023).
Pang, H. et al. Glymphatic function from diffusion-tensor MRI to predict conversion from mild cognitive impairment to dementia in Parkinson’s disease. J. Neurol. 271, 8 (2024).
Yao, J. et al. Early detection of dopaminergic dysfunction and glymphatic system impairment in Parkinson’s disease. Parkinsonism Relat. Disord. 127, 107089 (2024).
Gui, Q. et al. Relationship of glymphatic function with cognitive impairment, sleep disorders, anxiety and depression in patients with Parkinson’s disease. Neuropsychiatr. Dis. Treat. 20, 1809–1821 (2024).
Postuma, R. B. et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 142, 744–759 (2019).
Berg, D. et al. MDS research criteria for prodromal Parkinson’s disease: MDS Criteria for Prodromal PD. Mov. Disord. 30, 1600–1611 (2015).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines: PD-MCI Diagnostic Criteria. Mov. Disord. 27, 349–356 (2012).
Campabadal, A. et al. Disruption of posterior brain functional connectivity and its relation to cognitive impairment in idiopathic REM sleep behavior disorder. NeuroImage Clin. 25, 102138 (2020).
Campabadal, A. et al. Cortical gray matter and hippocampal atrophy in idiopathic rapid eye movement sleep behavior disorder. Front Neurol. 10, 312 (2019).
Mala, C. et al. Cortical and subcortical morphometric changes and their relation to cognitive impairment in isolated REM sleep behavior disorder. Neurol. Sci. 45, 613–627 (2024).
Zhang, X. et al. Cognitive and motor profiles as prodromal markers in predicting phenoconversion and phenotype in isolated REM sleep behavior disorder. Sleep. Med. 112, 262–272 (2023).
Génier Marchand, D. et al. How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann. Neurol. 83, 1016–1026 (2018).
Joza, S. et al. Prodromal dementia with Lewy bodies in REM sleep behavior disorder: A multicentric study. Brain J. Neurol. 146, 3258–3272 (2023).
Marchand, D. G., Montplaisir, J., Postuma, R. B., Rahayel, S. & Gagnon, J. F. Detecting the cognitive prodrome of dementia with lewy bodies: a prospective study of REM sleep behavior disorder. Sleep 40, zsw014 (2017).
Iranzo, A. et al. Significance of hyposmia in isolated REM sleep behavior disorder. J. Neurol. 268, 963–966 (2021).
Chae, J., Choi, M., Choi, J. & Yoo S. J. The nasal lymphatic route of CSF outflow: implications for neurodegenerative disease diagnosis and monitoring. Anim. Cells Syst. 28, 45–54 (2024).
Yoon, J. H. et al. Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage. Nature 625, 768–777 (2024).
Nandipati, S. & Litvan, I. Environmental exposures and Parkinson’s disease. Int. J. Environ. Res. Public Health 13, 881 (2016).
Chambers-Richards, T., Su, Y., Chireh, B. & D’Arcy, C. Exposure to toxic occupations and their association with Parkinson’s disease: a systematic review with meta-analysis. Rev. Environ. Health 38, 65–83 (2023).
Campabadal, A. et al. Comparing the accuracy and neuroanatomical correlates of the UPSIT-40 and the Sniffin’ Sticks test in REM sleep behavior disorder. Parkinsonism Relat. Disord. 65, 197–202 (2019).
Donzuso, G. et al. Neuroanatomical findings in isolated REM sleep behavior disorder and early Parkinson’s disease: a Voxel-based morphometry study. Brain Imaging Behav. 18, 83–91 (2024).
Iranzo, A. The REM sleep circuit and how its impairment leads to REM sleep behavior disorder. Cell Tissue Res. 373, 245–266 (2018).
Pardo, J. et al. Cortical Macro- and Microstructural Changes in Parkinson’s disease with probable rapid eye movement sleep behavior disorder. Mov. Disord. Off. J. Mov. Disord. Soc. 39, 814–824 (2024).
Siow, T. Y. et al. Association of sleep, neuropsychological performance, and gray matter volume with glymphatic function in community-dwelling older adults. Neurology 98, e829–e838 (2022).
Scott-Massey, A. et al. Glymphatic system dysfunction and sleep disturbance may contribute to the pathogenesis and progression of Parkinson’s disease. Int. J. Mol. Sci. 23, 12928 (2022).
Bohnen, N. I. & Hu, M. T. M. Sleep disturbance as potential risk and progression factor for Parkinson’s Disease. J. Park Dis. 9, 603–614 (2019).
Ferini-Strambi, L. et al. Autonomic symptoms in idiopathic REM behavior disorder: A multicentre case-control study. J. Neurol. 261, 1112–1118 (2014).
McCarter, S. J. et al. Autonomic dysfunction and phenoconversion in idiopathic REM sleep behavior disorder. Clin. Auton Res. 30, 207–213 (2020).
Elliott, J. et al. Frequency of orthostatic hypotension in isolated REM sleep behavior disorder: the north american prodromal synucleinopathy cohort. Neurology. 101, e2545–e2559 (2023).
Terzaghi, M. et al. Twenty-four-hour blood pressure profile in idiopathic REM sleep behavior disorder. Sleep 45, zsab239 (2022).
Zhong, G., Bolitho, S., Grunstein, R., Naismith, S. L. & Lewis, S. J. G. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson’s disease. PLoS ONE 8, 1–6 (2013).
Winer, J. R. et al. Isolated REM sleep behavior disorder is associated with altered 24-h rest-activity measures. Front. Sleep. 2, 1286124 (2023).
Feng, H. et al. Rest-activity pattern alterations in idiopathic REM sleep behavior disorder. Ann. Neurol. 88, 817–829 (2020).
Liguori, C. et al. Sleep−wake cycle dysregulation in idiopathic REM sleep behaviour disorder. J. Sleep. Res. 30, 1–6 (2021).
Filardi, M. et al. Objective rest–activity cycle analysis by actigraphy identifies isolated rapid eye movement sleep behavior disorder. Eur. J. Neurol. 27, 1848–1855 (2020).
Raupach, A. K. et al. Assessing the role of nocturnal core body temperature dysregulation as a biomarker of neurodegeneration. J. Sleep. Res. 29, 1–8 (2020).
Weissová, K. et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep. Med. 52, 1–6 (2018).
Wu, J. Q., Li, P., Stavitsky Gilbert, K., Hu, K. & Cronin-Golomb, A. Circadian rest-activity rhythms predict cognitive function in early Parkinson’s disease independently of sleep. Mov. Disord. Clin. Pr. 5, 614–619 (2018).
Berganzo, K. et al. Nocturnal hypertension and dysautonomia in patients with Parkinson’s disease: Are they related?. J. Neurol. 260, 1752–1756 (2013).
Videnovic, A. et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 71, 463–469 (2014).
Chen, Y. C., Wang, W. S., Lewis, S. J. G. & Wu, S. L. Fighting against the clock: circadian disruption and Parkinson’s disease. J. Mov. Disord. 17, 1–14 (2024).
Hablitz, L. M. et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 11, 4411 (2020).
Dubois, B. et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov. Disord. J. Mov. Disord. Soc. 22, 2314–2324 (2007).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies. Neurology 89, 88–100 (2017).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. J. Mov. Disord. Soc. 23, 2129–2170 (2008).
Cummings, J. L. et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314 (1994).
Beck, A. T., Steer, R. A. & Brown, G. Beck Depression Inventory–II. 1996.
Starkstein, S. E. et al. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 4, 134–139 (1992).
Aguirre-Mardones, C. et al. Prevalence and timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder. J. Neurol. 262, 1568–1578 (2015).
Lezak, M. D., Howieson, D. B., Bigler, E. D. & Tranel, D. Neuropsychological Assessment, 5th Ed. Oxford University Press; 2012:xxv, 1161.
Doty, R. L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 8, 329–339 (2012).
Martín-Barceló, C. et al. DTI-ALPS index: evaluation of several approaches for automatic computation. In: European Society for Magnetic Resonance in Medicine and Biology Congress 2024. ; 2024. https://www.esmrmb2024.org/abstracts-form/posters-e/abstract-data/d142b243414d6b4b3ca00758e68500df.
Acknowledgements
This study was sponsored by the Spanish Ministry of Economy and Competitiveness (MINECO PID2020-114640GB-I00/AEI/10.13039/501100011033 and PID2023-146932NB-I00 financed by MICIU/AEI/110.13039/5011000111033/FEDER,UE to C.J. and B.S.), Generalitat de Catalunya (SGR 2021SGR00801) and supported by María de Maeztu Unit of Excellence (Institute of Neurosciences, University of Barcelona) CEX2021-001159-M, Ministry of Science and Innovation. I.R. was supported by a fellowship from La Caixa Foundation (LCF/BQ/DR22/11950012). J.P. was supported by a fellowship from the Ministry of Science and Innovation (PRE2021-099674). C.M.B. was supported by María de Maeztu Unit of Excellence CEX2021-001159-M/ financed by MICIU/AEI/10.13039/501100011033. J.O. was supported by a fellowship from the Ministry of Science, Innovation, and Universities (PRE2018-086675). The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript. We would like to thank the cooperation of patients, their relatives, and healthy control participants. We are indebted to the Magnetic Resonance Imaging core facility of the FRCB-IDIBAPS for technical support. We would also like to acknowledge the CERCA Programme/Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
B.S. and C.J. contributed to the design of the study. I.R., J.P., A.C., J.O., G.M., A.M., A.I., C.G., M.S., C.P. contributed to data collection. I.R., C.M.-B. and C.F. contributed to data analysis. I.R., C.J.,A.I., and B.S. contributed to data interpretation. I.R. and B.S. contributed to the writing of the first draft, and C.J. and A.I. contributed to the final draft of the article. J.P., A.C., J.O., C.P., C.M.-B., C.F., N.B., C.G., G.M., A.M., A.I., C.J., M.B. and B.S. revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Roura, I., Pardo, J., Martín-Barceló, C. et al. Clinical and brain volumetric correlates of decreased DTI-ALPS, suggestive of local glymphatic dysfunction, in iRBD. npj Parkinsons Dis. 11, 87 (2025). https://doi.org/10.1038/s41531-025-00942-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-00942-z