Abstract
Predicting outcomes in individuals at clinical high risk (CHR) of developing psychosis remains challenging using clinical metrics alone. The PSYSCAN project aimed to enhance predictive value by integrating data across clinical, environmental, neuroimaging, cognitive, and peripheral blood biomarkers. PSYSCAN employed a naturalistic, prospective design across 12 sites (Europe, Australia, Asia, Americas). Assessments were conducted at baseline, 3, 6, and 12 months, with follow-ups at 18 and 24 months to evaluate clinical and functional outcomes. The study included 238 CHR individuals and 134 healthy controls (HC). At baseline, CHR and HC groups differed significantly in age, education, IQ, and vocational and relationship status. Cannabis and tobacco use did not significantly differ between groups, however CHR individuals had higher proportion of moderate to high risk of tobacco abuse. A substantial portion of the CHR sample met DSM criteria for anxiety (53.4%) and/or mood disorders (52.9%), with some prescribed antidepressants (38.7%), antipsychotics (13.9%), or benzodiazepines (16.4%). Over the follow-up period, 25 CHR individuals (10.5%) transitioned to psychosis. However, the CHR group as a whole showed improvements in functioning and attenuated psychotic symptoms. Similar to other recent multi-centre studies, the CHR cohort exhibits high comorbidity rates and relatively low psychosis transition rates. These findings highlight the clinical heterogeneity within CHR populations and suggest that outcomes extend beyond psychosis onset, reinforcing the need for broader prognostic models that consider functional and transdiagnostic outcomes.
Similar content being viewed by others
Introduction
Psychotic disorders usually emerge in late adolescence and early adulthood and can be personally and socially devastating due to the potential for life-long disability1,2,3. This has led to a worldwide effort to develop strategies for early identification, intervention, and prevention of psychosis4,5,6,7,8. A critical step towards this goal has been the operationalization of a “clinical high risk” (CHR) state9. People with this clinical syndrome typically present with attenuated symptoms, or less commonly, a brief psychotic episode that spontaneously resolves and/or genetic vulnerability in the context of a recent decline in functioning or chronic low functioning10. In addition to psychosis-related features, CHR individuals often exhibit a heterogeneous and complex clinical profile. Up to three-quarters have at least one comorbid mental health disorder, most commonly anxiety and mood disorders, but also including trauma-related and personality disorders11. Substance use is also prevalent in this group, with tobacco and cannabis being the most frequently reported substances12,13,14. The risk of developing psychosis among people presenting with a CHR state is high, and can vary from 12 to 43%, according to the nature of the sample, how it was ascertained, and the length of follow up15. However, most transitions occur during the first two years16. There is also a growing interest towards other outcomes, such as symptom severity17,18,19 and socio-occupational functioning impairments20. Although evidence is mixed, improvements in these areas are often seen within the first two years but are typically not sustained over the longer term21. Among the majority who do not develop psychosis, many already meet criteria for another mental health disorder or will subsequently experience other mental health issues or mental-health-related disability22,23.
Despite the heterogeneity of clinical presentation and outcomes, meeting CHR status remains the most reliable risk factor for psychosis24. This underscores the importance of studying the CHR state not only as a predictor of psychosis but also as a critical period for understanding the early alterations associated with the disorder. However, implementing such studies faces significant challenges. The low prevalence of CHR and transition rates in the general population compared to specialised CHR services25,26, coupled with the growing yet still limited availability of such services globally27, pose significant obstacles to recruitment. Furthermore, the large sample needed to disentangle specific pathways to psychosis onset in a heterogeneous group28 is difficult to achieve in single-centre studies within a reasonable timeframe. The inherent longitudinal nature of CHR studies adds further complexity. In response to these challenges, large-scale, multicentre, longitudinal studies such as NAPLS5, PRONIA4, and the ongoing AMP SZ29 have been developed. These collaborative efforts are designed to overcome the limitations of single-centre studies by pooling resources, harmonising methodologies, and increasing sample sizes to advance the understanding of CHR populations and improve early intervention strategies.
The PSYSCAN project is an international, naturalistic, prospective study focused on individuals at the early stages of psychosis. It encompasses a broad range of neuroimaging, clinical, cognitive, biological, and genetic variables collected at baseline and at follow-up30. CHR patients have been recruited from diverse regions, namely Europe, North America, South America, Asia, and Australia. This global reach captures variations in healthcare systems, cultural contexts and patterns of help-seeking, essential for advancing early detection and prevention of psychosis. This complements other large studies such as NAPLS that was carried out in one country5. For example, the NAPLS risk calculator to transition to psychosis6 generalised well to other North American sites31 but had a modest performance in a sample from Shanghai32. In addition to a wide geographical coverage, the PSYSCAN’s protocol includes a novel brief computerised cognitive battery for psychosis33, advanced neuroimaging techniques such as diffusion tensor imaging, and omics data to investigate less explored biomarkers in CHR such as keratinocytes, lipidomics, and redox. A further issue of past large studies in the early psychosis population is low retention rates. To overcome this issue and improve the quality and quantity of follow up data, the PSYSCAN protocol included close assessment time-points. The PSYSCAN study comprises three cohorts: individuals with a recent first episode of psychosis34, CHR and healthy controls (HC). The present paper describes how the CHR and healthy control samples were recruited and assessed, their sociodemographic and clinical characteristics at baseline, and the clinical and functional outcomes at follow up.
Material and methods
Study design and samples
A multi-centre, naturalistic, longitudinal design was employed. CHR individuals were assessed at baseline, 3, 6, 12, 18, and 24 months (Supplementary Table S1). The PSYSCAN project also involved the recruitment of a first episode psychosis patients (FEP) cohort, which is described in a separate study34, with healthy controls (HC) serving as a shared control group for both the CHR and FEP cohorts. HCs were assessed at baseline, 6, and 12 months (Supplementary Table S2).
CHR individuals were recruited from July 2016 to December 2019 at 10 sites: London, Amsterdam, Maastricht, Madrid, Naples, Melbourne, Seoul, Hong Kong, Toronto, Sao Paulo. The recruiting sites were all experienced in providing health care for CHR individuals. All participants were help-seeking and were under the care of a clinical service at the time of inclusion. A subset of 8 sites involved in the larger study (i.e. including FEP recruiting sites), additionally recruited HC individuals: London, Edinburgh, Utrecht, Maastricht, Amsterdam, Madrid, Seoul, and Melbourne. HC were recruited through advertisement and attempts were made to include HC who were similar in terms of sociodemographic characteristics (age, sex, and ethnicity) to the CHR and FEP cohorts.
Inclusion criteria for the CHR and HC cohorts were: age 16–40 years (except for one site, Madrid, who recruited 14–40), and ability to provide written informed consent, assent and written informed consent of parents and/or legal guardians if 14–17 years old (depending on local laws and regulations). The age lower limit was set to 16 as it aligns with research consent practices across sites. The broader upper age limit accommodates the inclusion of healthy controls shared with the FEP cohort, while the lower minimum age in Madrid results from the local collaboration between paediatric and adult psychiatry services. Additionally, CHR participants were required to meet criteria for either the Comprehensive Assessment of At-Risk Mental State (CAARMS) criteria9 or basic symptoms assessed using the Schizophrenia Proneness Instrument (SPI-A)35 (see Supplementary Materials for details). Extensive measures were undertaken to ensure consistency in clinical evaluations across sites using either CAARMS or SPI-A including training and monthly monitoring calls to discuss cases with experienced raters and clinicians30.
Exclusion criteria for CHR and HC included any previous neurosurgery or neurological disorder, including epilepsy; history of head injury resulting in unconsciousness lasting at least 1 hour; pregnancy, any other contraindications for MRI; refusing to have blood drawn and/or MRI performed; inability to fully comprehend the purpose of the study or make a rational decision whether or not to participate; having an estimated IQ < 70; having received antipsychotic medication for > 30 days (cumulative number of days) in the 3 months prior to baseline assessments (including self-ratings and screening assessments), at doses that would be adequate for treating a first episode of psychosis (i.e. excludes very low doses); a past episode of frank psychosis lasting > 7 days. In addition, HCs were excluded if they reported a lifetime history of any DSM-IV Axis-I or Axis-II (borderline, paranoid and schizotypal) disorder; met CAARMS9 or SPI-A35 criteria; had a first-degree relative with a lifetime history of affective or non-affective psychosis (defined by treatment or diagnosis); or reported previous use of antipsychotic medication or current use of any psychoactive medication. Ethical approval was obtained from each site’s local research ethics committee. The study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Assessments and measures
The comprehensive schedule of assessments, which were translated in each participating site’s main language, is listed in supplementary Table S1 (CHR) and S2 (HC). Both CHR individuals who did and did not develop psychosis followed the same assessment schedule. Those individuals who developed psychosis during the follow-up period were approached to complete all the planned visits listed in supplementary Table S1 and were not included in the FEP sample. Due to the start of the COVID-19 pandemic, assessments in 2020-21 were mostly completed online using telephone, or other remote platforms. To maximize retention and data completeness, follow-up assessments were extended beyond 24 months when feasible and/or necessary. This occurred when (1) COVID-19 delays disrupted scheduled assessments, (2) participants missed their 24-month follow-up but agreed to later contact, or (3) they remained engaged with clinical services beyond 24 months. In these cases, data were collected via face-to-face assessments or electronic health records.
Sociodemographic data were collected at baseline. Medical and psychiatric history were also evaluated at baseline and updated during subsequent assessments using a semi-structured interview. Psychopathology in CHR individuals was assessed at baseline, 6, 12, and 24 months using a two-part tool (Clinical High Risk Assessment Tool; CHRA, Part 1 and Part 2). Additionally, interim assessments at 3 and 18 months were conducted to determine if the individual had transitioned to psychosis. CHR state, symptoms remission (defined as no longer meeting criteria for CHR state) and transition to psychosis during the study were assessed using the Psychotic Symptoms module of the CAARMS9 and SPI-A)35. Functioning was assessed using Social and Occupational Functioning Scale (SOFAS)36. Criteria for other mental health disorders were assessed using the Structured Clinical Interview for DSM-IV Disorders -I (SCID-I37) and SCID-II (paranoid, schizotypal, and borderline personality disorder modules)38). Inter-rater reliability and consistency across sites were ensured throughout via monthly or bimonthly group monitoring calls where new cases were discussed and CAARMS9 and SPI-A35 scoring confirmed by experienced clinicians and trainers.
Substance use was assessed with the Alcohol, Smoking and Substance Involvement Screening Test 3.0 (ASSIST39). Cannabis use, age of onset, frequency, quantity, duration and substance preference was assessed using the adapted Cannabis Experience Questionnaire40. IQ was assessed using a short version of the WAIS41.
Transition to psychosis was defined either psychometrically using CAARMS criteria or clinically using Diagnostic and Statistical Manual of Mental Disorders (DSM)- or the International Classification of Diseases (ICD)-defined diagnoses or accepted referrals to early intervention services recorded in electronic health records (EHRs).
Statistical analysis
All data collected were transferred to a central database managed by IXICO (https://ixico.com) for processing and analysis throughout the project. The present study focuses on the analyses on key baseline socio-demographic in the CHR and HC samples and clinical data in the CHR sample, as well as follow up data on CHR functioning and symptom severity. Data analysis was performed using python 3.5 and R 4.2.2. The threshold for statistical significance was p < 0.05. Independent samples t-tests and Chi-square tests were used to compare CHR and HC on continuous and categorical variables, respectively. Symptom severity was indexed by multiplying severity and frequency scores for each of the four CAARMS positive items. The total CAARMS positive score was the sum of these individual CAARMS positive items. Functional remission was defined as a SOFAS score >68 as defined in previous studies42. The cumulative incidence of psychosis was visualized with the Kaplan–Meier failure function (1—survival)43 and Greenwood 95% confidence intervals (CIs)44, conducted using the “survival” (version 3.5-7) and “survminer” (version 0.4.9) packages. The difference in incidence between individuals followed up using psychometric assessment (CAARMS) and EHRs was compared using a Cox proportional hazards model, following confirmation of the proportional hazards assumption being met using the Global Schoenfeld Test45. Cox proportional hazards models were run both unadjusted and adjusted for sociodemographic (age, sex and ethnicity) or clinical variables (baseline CAARMS positive scores and baseline SOFAS scores) with significant group differences (assessed with independent t-test for continuous variables and Fisher’s exact test for categorical variables). Differences between baseline and follow-up positive CAARMS and SOFAS scores were assessed using paired two-tailed t-tests. To mitigate against potential survivorship bias, we presented the baseline descriptives for all participants and then compared the difference between scores from the baseline assessment and each participant’s final follow-up assessment, only in participants who attended at least one follow-up assessment.
Results
Recruitment and retention
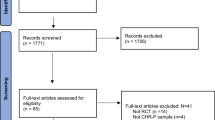
From July 2016 to December 2019, 372 participants were recruited across the 12 sites. This included 238 CHR and 134 HC (Table 1). CHR participants were recruited from 10 different sites, with a mean of 23.8 subjects enrolled per site (Fig. 1). In general, recruitment was highest at sites where there was a well-established clinical early detection service. The HC sample was recruited from 8 sites, with a mean of 16.8 subjects enrolled per site.
77.9% of the CHR sample were followed-up at 3 months, 75.0% at 6 months, 61.4% at 12 months, 55.0% at 18 months and 50.4% at the final 24-month assessment (Fig. S1). For the HC sample, 74.2% completed the 6-month follow-up assessment and 73.5% the 12-month assessment (Fig. S2). If the COVID-19 pandemic disrupted follow-up assessments, they were conducted remotely instead of in person.
Socio-demographic characteristics
We aimed to recruit a HC sample that would be socio-demographically similar to the CHR sample, but the groups slightly differed in age (23.7 versus 22.4), years in education (15.9 versus 14.1 for CHR) and estimated IQ (112.6 versus 105.0). HC were also more likely than CHR participants to be in education (64.1% versus 48.7%) or/and employment (77.1% versus 49.7%), and to be in a relationship (43.0% versus 23.7%) (Table 1).
Clinical characteristics
In total, 228 CHR participants met CAARMS CHR criteria, 117 met Basic Symptoms criteria and 107 met both (Table 2, Fig. 2). Of the 228 participants that met the CAARMS inclusion criteria, 215 (94.3%) had attenuated psychotic symptoms, while 41 (18.0%) had trait liability (SPD or a first-degree relative with psychosis) and functional decline, and 20 (8.8%) had BLIPS. Most of the subjects with trait liability and BLIPS also had attenuated symptoms: only 11 of 228 participants who met the CAARMS criteria did so on the basis of genetic risk or BLIPS alone. Ten participants (4.2%) met Basic Symptoms criteria only.
The mean baseline SOFAS score for all participants was 53.7 (SD = 11.4). Mean baseline CAARMS severity scores (severity*frequency scores) for all participants across the four psychosis items were 11.4 (SD = 9.4) for unusual thought content, 13.4 (SD = 9.3) for non-bizarre ideas, 10.9 (SD = 7.1) for perceptual abnormalities, 6.8 (SD = 6.6) for disorganised speech, and 42.5 (SD = 20.3) for the total CAARMS positive.
Many CHR individuals also met DSM-IV criteria for an anxiety disorder (53.4%) or for a mood disorder (52.9%) (Table 2, Fig. S3), far fewer met criteria for an eating (8.4%) or a somatoform disorder (4.6%). Over a third of the CHR sample (38.7%) were taking antidepressant medications. A minority were taking antipsychotic medications (13.9%) or benzodiazepines (16.4%).
There were no significant differences in substance use between HC and CHR over the previous 3 months or the participant’s lifetime, except that HC individuals (89.9%) were more likely to have reported alcohol use in the last 3 months compared with CHR (73.7%). However, there was a higher proportion of moderate risk for tobacco abuse in CHR (46.0%) than HC (35.1%) (Table 3).
The most frequent sources of referral were community mental health teams (47.2%), social services or supported accommodation (15.0%) and general practitioner (14.0%) (Supplementary Table S3).
Follow-up rates and transition status
Among the 238 CHR participants recruited to the study, 202 (84.9%) participated in at least one follow-up assessment and EHRs (available from nine sites) were accessed for 209 (87.8%) participants. Using these two data sources, follow-up data were available for 224 (94.1%) of CHR individuals, followed for a mean of 691.6 (SD = 438.1) days.
In total, 25 (10.5%) CHR individuals developed a FEP; 16 of these transitions (64.0%) were defined psychometrically using the CAARMS and nine (36.0%) were defined clinically through clinical data in EHRs (see Table S4 for a descriptive comparison of demographic and clinical characteristics between those who transitioned and those who did not). The cumulative incidence of psychosis was 0.019 (95%CI: 0.000-0.038, 196 individuals still at risk) at 6 months, 0.051 (95%CI: 0.020–0.082, 169 individuals still at risk) at 12 months, 0.087 (95%CI: 0.045–0.127, 133 individuals still at risk) at 18 months and 0.111 (95%CI: 0.062–0.158, 102 individuals still at risk) at 24 months, 0.170 (95%CI: 0.092–0.240, 37 individuals still at risk) at 36 months, 0.170 (95%CI: 0.092–0.240, 15 individuals still at risk) at 48 months, 0.377 (95%CI: 0.062-0.587, 3 individuals still at risk) at 60 months, 0.377 (95%CI: 0.062–0.587, 2 individuals still at risk) at 72 months (Fig. 3).
EHR-based follow-up was associated with a lower risk of transition compared to those followed-up using the CAARMS (HR = 0.34, 95%CI: 0.13–0.87, p = 0.025; Fig. 3). There was a significantly lower proportion of female participants represented in EHR follow-up compared to those followed up using the CAARMS alone (p = 0.04) and differences in ethnicity (p < 0.001), largely driven by lower proportions of Asian participants and higher proportions of Black participants in EHR follow-up. There were no differences in age, baseline CAARMS or baseline SOFAS scores between the two groups (p > 0.05). The difference in transition risk between EHR- and CAARMS-based follow-up was no longer significant when adjusted for sex and ethnicity (adjusted hazard ratio, aHR=0.47, 95%CI: 0.17–1.34, p = 0.16).
Symptomatic and functional outcomes at follow-up
Clinical assessments were available for 202 CHR participants who attended at least one follow-up assessment; the mean time between the baseline and the last observed clinical assessment for these participants was 546 (SD = 325.9) days. After excluding those who transitioned to psychosis (irrespective of data source), 78/180 (43.3%) CHR individuals continued to meet CHR status at their final clinical follow-up whilst 102/180 (56.7%) showed remission from the CHR state.
Of those who attended at least one follow-up assessment, 19/202 (9.4%) CHR individuals had clinically determined good functioning at baseline and 80/202 (39.6%) at their last follow-up visit. There was a significant increase in SOFAS scores from 53.6 to 64.4 (mean difference = 10.9, 95%CI: 9.0–12.9, p < 0.001). Similarly, there was a significant decrease in positive symptom severity across all CAARMS positive items (unusual thought content [mean difference = 7.1, 95%CI: 5.7–8.5, p < 0.001]; non-bizarre ideas [mean difference = 7.1, 95%CI: 5.6–8.6, p < 0.001]; perceptual abnormalities [mean difference = 5.8, 95%CI:4.7-7.0, p < 0.001]; disorganised speech [mean difference = 2.9, 95%CI:2.0–3.9, p < 0.001]) and total CAARMS positive (mean difference = 23.0, 95%CI: 19.4–26.5, p < 0.001).
Discussion
A large sample of 238 CHR for psychosis and 134 HC were recruited and included into the PSYSCAN study. A comprehensive number of clinical measures were collected at baseline and 5 follow-up timepoints generating a rich and well-characterised dataset. The sample was characterised by a high prevalence of psychiatric comorbidities, with more than half of CHR participants presenting with a baseline diagnosis of an anxiety or mood disorder. The pattern of comorbidities was highly heterogeneous, also including post-traumatic stress disorder, eating disorders, and somatoform disorders. This aligns with cumulative evidence demonstrating that the CHR population is diverse in its clinical presentation11. It also supports the notion that psychosis onset may emerge from various non-psychotic precursors46, highlighting its inherently transdiagnostic nature. As a result, there have been recent calls to expand CHR assessments to include baseline evaluations of comorbid psychopathological dimensions11 and the development of early detection services for young people who are vulnerable to a range of adult psychiatric disorders, rather than just psychosis47. However, broadening the inclusion criteria for early detection teams can substantially increase the logistical demands on these clinical services, with much greater numbers of potential referrals48. The high prevalence of MDD among CHR individuals, particularly its greater prevalence in those who transitioned to psychosis compared to those who did not, is consistent with evidence suggesting that a history of depressive episodes may adversely affect the course of attenuated psychotic symptoms in CHR49. This aligns with the affective pathway to psychosis, which posits that affective dysregulation acts as the central link between early traumatic or stressful experiences and the onset of psychosis14,50,51.
There was a lack of significant differences in both recent and lifetime cannabis use between CHR subjects and HC. While this is in line with other studies14, it contrasts with the higher rates of cannabis use in people who have developed a psychotic disorder, which have consistently been found to be higher than in controls52. This difference in cannabis use between CHR and psychotic samples raises the possibility that in people with psychosis, cannabis use may partly be driven by effects of the disorder itself. Although cannabis use has been implicated as a risk factor for psychosis53, recent follow up studies in CHR samples have not found a significant association between cannabis use and later transition to psychosis54,55,56.
Over 90% of the CHR individuals were followed up either face to face or remotely. The rate of transition to psychosis was relatively low (10.5%), but similar to that in other recent multi-centre studies57,58. Previous meta-analytical work suggests that transition to psychosis in CHR samples may be declining, possibly due to improved clinical engagement, active interventions, and early detection59. This presents both a conceptual and analytical challenge, as the relatively low rate of transition limits the statistical power to identify predictors of psychotic disorder as an outcome. Potential approaches to address this issue include exploring long-term transition risk15, investigating transdiagnostic outcomes such as socio-occupational functioning60, or broadening the transdiagnostic inclusion criteria for psychosis-risk populations61.
In previous prospective studies of CHR samples and in a recent meta-analysis23, there was an overall improvement in both symptom severity and level of functioning subsequent to baseline. Among those who did not develop psychosis, a substantial proportion (43.3%) had not achieved symptomatic remission (defined as no longer meeting criteria for the CHR state) at the last available follow-up, or had not shown an improvement in their level of functioning (39.6%)42. Thus, despite a group-level improvement in clinical and functional status over time, a large proportion of our CHR sample that did not become psychotic had poor clinical and/or functional outcomes.
Strengths and limitations
This study has several strengths. Firstly, we recruited a relatively large sample of well-characterised CHR and HC, retaining a large proportion of them into the study across multiple follow-ups. PSYSCAN participants were recruited across 12 sites located across four continents. CHR participants can be difficult to recruit to research studies. Multi-centre studies like PSYSCAN provide a way to enrol large CHR samples but are logistically demanding and require substantial funding. In the present study, retention rates for CHR were satisfactory, with up to 61% completing the 12-month follow-up assessment and 50% completing follow up at 24 months (or later). This may have been due to the higher frequency of follow-up assessments compared to previous large studies62 as well as the use of EHR to supplement missing data.
There are also several limitations. Firstly, some sites recruited a small number of participants, introducing the potential for confounding site effects30. Many centres do not have clinical early detection services for CHR individuals, and within the PSYSCAN consortium, sites that lacked this specialised infrastructure recruited significantly fewer participants. Even when such services are well-established, CHR individuals may be referred to other clinical teams63. In the present study, we sought to minimise these effects by standardising assessments and protocols, and regularly training study researchers in their use. Secondly, proportion who transitioned to psychosis was relatively small (10%), although in line with similar large studies57,58. While this makes the prediction of psychosis transition difficult to analyse, we do have a well characterised group of CHR and it will be possible to assess how accurately other important outcomes such as symptom remission and functioning can be predicted from baseline multimodal data. Nevertheless, the inability to assess individuals lost to follow-up may introduce potential bias in subsequent analyses to identify predictors of outcomes. Finally, despite the onset of COVID-19 pandemic during the follow up phase of the study, most sites were able to continue with clinical assessments by conducting these remotely instead of face-to-face. However, delays in obtaining approval to conduct the assessments remotely during this period likely extended assessments dates for some participants. Additionally, the stress and uncertainty associated with the pandemic could have exacerbated symptoms of psychosis and general psychopathology64, potentially impacting the clinical measures captured during this period.
Conclusions
Consistent with other recent multi-centre studies, the PSYSCAN CHR cohort is characterised by high levels of psychiatric comorbidity and relatively low rates of transition to psychosis. The core aim of the PSYSCAN study is to integrate neuroimaging, clinical, cognitive, and peripheral biomarker data to facilitate the prediction of clinical and functional outcomes in CHR individuals. A large sample of individuals at CHR for psychosis and HC was recruited and assessed at multiple time points. The study has generated a multi-modal dataset that will be used to identify predictors of outcomes in this population.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Wittchen, H. U. et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 21, 655–679 (2011).
Fusar-Poli, P. et al. The lived experience of psychosis: a bottom-up review co-written by experts by experience and academics. World Psychiatry 21, 168–188 (2022).
Estrade, A. et al. The Lived Experiences of Family Members and Carers of People with Psychosis: A Bottom-Up Review Co-Written by Experts by Experience and Academics. Psychopathology 56, 371–382 (2023).
Koutsouleris, N. et al. Prediction Models of Functional Outcomes for Individuals in the Clinical High-Risk State for Psychosis or With Recent-Onset Depression: A Multimodal, Multisite Machine Learning Analysis. JAMA Psychiatry, https://doi.org/10.1001/jamapsychiatry.2018.2165 (2018).
Addington, J. et al. North American Prodrome Longitudinal Study (NAPLS 3): Methods and baseline description. Schizophr Res. https://doi.org/10.1016/j.schres.2020.04.010 (2020).
Cannon, T. D. et al. An Individualized Risk Calculator for Research in Prodromal Psychosis. Am. J. Psychiatry 173, 980–988 (2016).
Fusar-Poli, P. et al. Development and Validation of a Clinically Based Risk Calculator for the Transdiagnostic Prediction of Psychosis. JAMA Psychiatry 74, 493–500, (2017).
Oliver, D. et al. Transdiagnostic individualized clinically-based risk calculator for the automatic detection of individuals at-risk and the prediction of psychosis: external replication in 2,430,333 US patients. Transl. Psychiatry 10, 364 (2020).
Yung, A. R. et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust. N. Z. J. Psychiatry 39, 964–971 (2005).
Yung, A. R. & McGorry, P. D. The Prodromal Phase of First-Episode Psychosis: Past and Current Conceptualizations. Schizophr. Bull. 22, 353–370 (1996).
Solmi, M. et al. Meta-analytic prevalence of comorbid mental disorders in individuals at clinical high risk of psychosis: the case for transdiagnostic assessment. Mol. Psychiatry 28, 2291–2300 (2023).
Carney, R., Cotter, J., Bradshaw, T., Firth, J. & Yung, A. R. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: A systematic review and meta-analysis. Schizophr. Res. 170, 290–300 (2016).
Carney, R., Cotter, J., Firth, J., Bradshaw, T. & Yung, A. R. Cannabis use and symptom severity in individuals at ultra high risk for psychosis: a meta-analysis. Acta Psychiatr. Scand. 136, 5–15 (2017).
Fusar-Poli, P. et al. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur. Psychiatry 40, 65–75 (2017).
Salazar de Pablo, G. et al. Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-analysis. JAMA Psychiatry 78, 970–978, (2021).
Kempton, M. J., Bonoldi, I., Valmaggia, L., McGuire, P. & Fusar-Poli, P. Speed of Psychosis Progression in People at Ultra-High Clinical Risk: A Complementary Meta-analysis. JAMA Psychiatry 72, 622–623, (2015).
DeVylder, J. E. et al. Assessing depression in youth at clinical high risk for psychosis: a comparison of three measures. Psychiatry Res. 215, 323–328 (2014).
Lencz, T., Smith, C. W., Auther, A., Correll, C. U. & Cornblatt, B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr. Res. 68, 37–48 (2004).
Calkins, M. E. et al. Concordance and factor structure of subthreshold positive symptoms in youth at clinical high risk for psychosis. Schizophr. Res. 227, 72–77 (2021).
Addington, J., Penn, D., Woods, S. W., Addington, D. & Perkins, D. O. Social functioning in individuals at clinical high risk for psychosis. Schizophr. Res. 99, 119–124 (2008).
Salazar de Pablo, G. et al. Longitudinal outcome of attenuated positive symptoms, negative symptoms, functioning and remission in people at clinical high risk for psychosis: a meta-analysis. EClinicalMedicine 36, 100909 (2021).
Michel, C., Ruhrmann, S., Schimmelmann, B. G., Klosterkotter, J. & Schultze-Lutter, F. Course of clinical high-risk states for psychosis beyond conversion. Eur. Arch. Psychiatry Clin. Neurosci. 268, 39–48 (2018).
Salazar de Pablo, G. et al. Clinical outcomes in individuals at clinical high risk of psychosis who do not transition to psychosis: a meta-analysis. Epidemiol. Psychiatr. Sci. 31, e9 (2022).
Oliver, D. et al. Exploring causal mechanisms of psychosis risk. Neurosci. Biobehav Rev. 162, 105699 (2024).
Fusar-Poli, P. et al. Deconstructing Pretest Risk Enrichment to Optimize Prediction of Psychosis in Individuals at Clinical High Risk. JAMA Psychiatry 73, 1260–1267, (2016).
Fusar-Poli, P. et al. Prevention of Psychosis: Advances in Detection, Prognosis, and Intervention. JAMA Psychiatry 77, 755–765, (2020).
Kotlicka-Antczak, M. et al. Worldwide implementation of clinical services for the prevention of psychosis: The IEPA early intervention in mental health survey. Early Inter. Psychiatry 14, 741–750 (2020).
Fusar-Poli, P. et al. Heterogeneity of Psychosis Risk Within Individuals at Clinical High Risk: A Meta-analytical Stratification. JAMA Psychiatry 73, 113–120, (2016).
Wannan, C. M. J. et al. Accelerating Medicines Partnership(R) Schizophrenia (AMP(R) SCZ): Rationale and Study Design of the Largest Global Prospective Cohort Study of Clinical High Risk for Psychosis. Schizophr. Bull. 50, 496–512 (2024).
Tognin, S. et al. Towards Precision Medicine in Psychosis: Benefits and Challenges of Multimodal Multicenter Studies-PSYSCAN: Translating Neuroimaging Findings From Research into Clinical Practice. Schizophr. Bull. 46, 432–441 (2020).
Carrion, R. E. et al. Personalized Prediction of Psychosis: External Validation of the NAPLS-2 Psychosis Risk Calculator With the EDIPPP Project. Am. J. Psychiatry 173, 989–996 (2016).
Zhang, T. et al. Validating the Predictive Accuracy of the NAPLS-2 Psychosis Risk Calculator in a Clinical High-Risk Sample From the SHARP (Shanghai At Risk for Psychosis) Program. Am. J. Psychiatry 175, 906–908 (2018).
Gifford, G. et al. PsyCog: A computerised mini battery for assessing cognition in psychosis. Schizophr. Res Cogn. 37, 100310 (2024).
Slot, M. I. E. et al. A naturalistic cohort study of first-episode schizophrenia spectrum disorder: A description of the early phase of illness in the PSYSCAN cohort. Schizophr. Res. 266, 237–248 (2024).
Schultze-Lutter, F., Ruhrmann, S., Picker, H. & Klosterkotter, J. Development and evaluation of the schizophrenia proneness instrument, adult version (SPI-A). Schizophr. Res. 86, S4–S5 (2006).
Goldman, H. H., Skodol, A. E. & Lave, T. R. Revising axis V for DSM-IV: a review of measures of social functioning. Am. J. Psychiatry 149, 1148–1156 (1992).
First, M. B., Spitzer, R. L., Givvon, M. & Williams, J. B. W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). (New York: Biometrics Research, 2002).
First, M. B., Gibbon, M., Spitzer, R. L., Williams, J. B. W. & Benjamin, L. S. Structured clinical interview for DSM-IV Axis II personality disorders (SCID-II). (American Psychiatric Press, Inc., 1997).
Group, W. A. W. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 97, 1183–1194 (2002).
van Laar, M., Frijns, T., Trautmann, F. & Lombi, L. Sizing the cannabis market: a demand-side and user-specific approach in seven European countries. Curr. Drug Abus. Rev. 6, 152–164 (2013).
Blyler, C. R., Gold, J. M., Iannone, V. N. & Buchanan, R. W. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr. Res. 46, 209–215 (2000).
Iorfino, F. et al. Delineating the trajectories of social and occupational functioning of young people attending early intervention mental health services in Australia: a longitudinal study. BMJ Open 8, e020678 (2018).
Kaplan, E. L. M. P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 53, 457 (1958).
Lazarus-Barlow, W. S. L. & Leeming, J. H. The natural duration of cancer. Br. Med. J. 2, 266–267 (1924).
Grambsch, P. M. & Therneau, T. M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81, 515–526 (1994).
Guloksuz, S. et al. Association of preceding psychosis risk states and non-psychotic mental disorders with incidence of clinical psychosis in the general population: a prospective study in the NEMESIS-2 cohort. World Psychiatry 19, 199–205 (2020).
Rickwood, D. et al. Sixteen years of innovation in youth mental healthcare: Outcomes for young people attending Australia’s headspace centre services. PLoS One 18, e0282040 (2023).
Solmi, M., Durbaba, S., Ashworth, M. & Fusar-Poli, P. Proportion of young people in the general population consulting general practitioners: Potential for mental health screening and prevention. Early Inter. Psychiatry 14, 631–635 (2020).
Schirmbeck, F. et al. Impact of Comorbid Affective Disorders on Longitudinal Clinical Outcomes in Individuals at Ultra-high Risk for Psychosis. Schizophr. Bull. 48, 100–110 (2022).
Klippel, A. et al. Modeling the Interplay Between Psychological Processes and Adverse, Stressful Contexts and Experiences in Pathways to Psychosis: An Experience Sampling Study. Schizophr. Bull. 43, 302–315 (2017).
Isvoranu, A. M. et al. A Network Approach to Psychosis: Pathways Between Childhood Trauma and Psychotic Symptoms. Schizophr. Bull. 43, 187–196 (2017).
Koskinen, J., Lohonen, J., Koponen, H., Isohanni, M. & Miettunen, J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr. Bull. 36, 1115–1130 (2010).
Di Forti, M. et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr. Bull. 40, 1509–1517 (2014).
Buchy, L. et al. Substance use in individuals at clinical high risk of psychosis. Psychol. Med. 45, 2275–2284 (2015).
Chester, L. A. et al. Influence of cannabis use on incidence of psychosis in people at clinical high risk. Psychiatry Clin. Neurosci. 77, 469–477 (2023).
Oliver, D. et al. What Causes the Onset of Psychosis in Individuals at Clinical High Risk? A Meta-analysis of Risk and Protective Factors. Schizophr. Bull. 46, 110–120 (2020).
Addington, J. et al. North American Prodrome Longitudinal Study (NAPLS 2): The Prodromal Symptoms. J. Nerv. Ment. Dis. 203, 328–335 (2015).
Koutsouleris, N. et al. Multimodal Machine Learning Workflows for Prediction of Psychosis in Patients With Clinical High-Risk Syndromes and Recent-Onset Depression. JAMA Psychiatry 78, 195–209, (2021).
Fusar-Poli, P. et al. Predicting Psychosis Meta-analysis of Transition Outcomes in Individuals at High Clinical Risk. Arch. Gen. Psychiatry 69, 220–229, (2012).
Fusar-Poli, P. et al. Disorder, not just state of risk: meta-analysis of functioning and quality of life in people at high risk of psychosis. Br. J. Psychiatry 207, 198–206 (2015).
McGorry, P. D., Hartmann, J. A., Spooner, R. & Nelson, B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry 17, 133–142 (2018).
Tognin, S. et al. Emotion Recognition and Adverse Childhood Experiences in Individuals at Clinical High Risk of Psychosis. Schizophr. Bull. 46, 823–833 (2020).
Salazar de Pablo, G., Woods, S. W., Drymonitou, G., de Diego, H. & Fusar-Poli, P. Prevalence of Individuals at Clinical High-Risk of Psychosis in the General Population and Clinical Samples: Systematic Review and Meta-Analysis. Brain Sci. 11, https://doi.org/10.3390/brainsci11111544 (2021).
Pereira-Sanchez, V. et al. COVID-19 effect on mental health: patients and workforce. Lancet Psychiatry 7, e29–e30 (2020).
Acknowledgements
We would like to thank all participants who gave their time and took part in the study. We would also like to thank all researchers who continued to work and collect data during the COVID-19 pandemic. The PSYSCAN Project is supported by grant agreement n° 603196 under the European Union’s Seventh Framework Programme. CA has been supported by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (ISCIII), co-financed by the European Union, ERDF Funds from the European Commission, “A way of making Europe”, financed by the European Union – Next Generation EU (PMP21/00051), PI19/01024. PI22/01824 CIBERSAM, Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), European Union Structural Funds, European Union Seventh Framework Program, European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking: Project PRISM-2 (Grant agreement No.101034377), Project AIMS-2-TRIALS (Grant agreement No 777394), Horizon Europe, the National Institute of Mental Health of the National Institutes of Health under Award Number 1U01MH124639-01 (Project ProNET) and Award Number 5P50MH115846-03 (project FEP-CAUSAL), Fundación Familia Alonso, and Fundación Alicia Koplowitz. BN was supported by an NHMRC Investigator Grant (2026484). SV was supported by the Sir Henry Wellcome Trust Postdoctoral Fellowship (221638/Z/20/Z).
Author information
Authors and Affiliations
Consortia
Contributions
S.T and S.V. contributed equally to this work (co-first authorship). ST: Conceptualization, Writing - Original Draft; SV: Writing - Original Draft, Formal analysis; DO: Writing - Original Draft, Formal analysis; AEC: Conceptualization, Data curation, Writing - Original Draft; All authors: Writing - Review & Editing.
Corresponding author
Ethics declarations
Competing interests
C.A. has been a consultant to or has received honoraria or grants from Abbot, Acadia, Ambrosetti, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, Takeda and Teva. SG received compensation for serving as a consultant or speaker, or she or the institutions she works for have received research support from the companies or organizations indicated: Angelini, Gedeon Richter-Recordati, Janssen Cilag, Lundbeck Italia, Medscape, Takeda, Rovi and Boehringer Ingelheim. All other authors have no competing interest to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tognin, S., Vieira, S., Oliver, D. et al. PSYSCAN multi-centre study: baseline characteristics and clinical outcomes of the clinical high risk for psychosis sample. Schizophr 11, 66 (2025). https://doi.org/10.1038/s41537-025-00598-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-025-00598-x