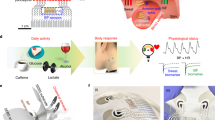
Abstract
The development of closed-loop systems towards effective management of diabetes requires the inclusion of additional chemical and physical inputs that affect disease pathophysiology and reflect cardiovascular risks in patients. Comprehensive glycaemic control information should account for more than a single glucose signal. Here, we describe a hybrid flexible wristband sensing platform that integrates a microneedle array for multiplexed biomarker sensing and an ultrasonic array for blood pressure, arterial stiffness and heart-rate monitoring. The integrated system provides a continuous evaluation of the metabolic and cardiovascular status towards improving glycaemic control and alerting patients to cardiovascular risks. The multimodal platform offers continuous glucose, lactate and alcohol monitoring, along with simultaneous ultrasonic measurements of blood pressure, arterial stiffness and heart rate, to support understanding of the interplay between interstitial fluid biomarkers and physiological parameters during common activities. By expanding the continuous monitoring of patients with diabetes to additional biomarkers and key cardiac signals, our integrated multiplexed chemical–physical health-monitoring platform holds promise for addressing the limitations of existing single-modality glucose-monitoring systems towards enhanced management of diabetes and related cardiovascular risks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The manuscript includes all the data collected and analysed in the study, and more information is included in the Supplementary Information.
Code availability
The code for decoding the transducer data to blood pressure waveforms is available via GitHub at https://github.com/MuyangLin95/Processing-scripts-for-monitoring-metabolites-and-cardiac-signals-in-diabetes.git (ref. 63).
References
Zimmet, P. Z., Magliano, D. J., Herman, W. H. & Shaw, J. E. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2, 56–64 (2014).
Roglic, G. WHO Global report on diabetes: a summary. Int. J. Noncommun. Dis. 1, 3–8 (2016).
Klonoff, D. C., Ahn, D. & Drincic, A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res. Clin. Pract. 133, 178–192 (2017).
Lin, R., Brown, F., James, S., Jones, J. & Ekinci, E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet. Med. 38, e14528 (2021).
Teymourian, H., Barfidokht, A. & Wang, J. Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem. Soc. Rev. 49, 7671–7709 (2020).
Wolkowicz, K. L. et al. A review of biomarkers in the context of type 1 diabetes: biological sensing for enhanced glucose control. Bioeng. Transl. Med. 6, e10201 (2020).
Avogaro, A. et al. Glucose tolerance during moderate alcohol intake: insights on insulin action from glucose/lactate dynamics. J. Clin. Endocrinol. Metab. 87, 1233–1238 (2002).
Zhou, M.-S., Wang, A. & Yu, H. Link between insulin resistance and hypertension: what is the evidence from evolutionary biology? Diabetol. Metab. Syndr. 6, 12 (2014).
Svensson, M. K. et al. Alterations in heart rate variability during everyday life are linked to insulin resistance. A role of dominating sympathetic over parasympathetic nerve activity? Cardiovasc. Diabetol. 15, 91 (2016).
Eckel, R. H., Bornfeldt, K. E. & Goldberg, I. J. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 33, 1519–1545 (2021).
Chen, Y., Lee, K., Ni, Z. & He, J. C. Diabetic kidney disease: challenges, advances, and opportunities. Kidney Dis. 6, 215–225 (2020).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 6, 1214–1224 (2022).
Tasca, F., Tortolini, C., Bollella, P. & Antiochia, R. Microneedle-based electrochemical devices for transdermal biosensing: a review. Curr. Opin. Electrochem. 16, 42–49 (2019).
Himawan, A. et al. Where microneedle meets biomarkers: futuristic application for diagnosing and monitoring localized external organ diseases. Adv. Healthc. Mater. 12, 2202066 (2023).
Imani, S. et al. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Xu, Y. et al. In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat. Nat. Biomed. Eng. 7, 1307–1320 (2023).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Xu, C. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 7, 168–179 (2024).
Bernardin, G., Pradier, C., Tiger, F., Deloffre, P. & Mattei, M. Blood pressure and arterial lactate level are early indicators of short-term survival in human septic shock. Intensive Care Med. 22, 17–25 (1996).
Ho, K. K. Y. et al. Evaluation of an anti-thrombotic continuous lactate and blood pressure monitoring catheter in an in vivo piglet model undergoing open-heart surgery with cardiopulmonary bypass. Chemosensors (Basel) 8, 56 (2020).
Moonla, C. et al. Continuous ketone monitoring via wearable microneedle patch platform. ACS Sens. 9, 1004–1013 (2024).
Roh, H. et al. Fabrication of high-density out-of-plane microneedle arrays with various heights and diverse cross-sectional shapes. Nanomicro. Lett. 14, 24 (2021).
Chang, A.-Y. et al. Dopamine sensing with robust carbon nanotube implanted polymer micropillar array electrodes fabricated by coupling micromolding and infiltration coating processes. Electrochim. Acta 368, 137632 (2021).
Römgens, A. M., Bader, D. L., Bouwstra, J. A., Baaijens, F. P. T. & Oomens, C. W. J. Monitoring the penetration process of single microneedles with varying tip diameters. J. Mech. Behav. Biomed. Mater. 40, 397–405 (2014).
MicroChem. SU-8 2000.5-2015 Permanent Epoxy Negative Photoresist PROCESSING GUIDELINES. SU-8 2000 Datasheet 1–5 (Kayaku, 2015).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Gordin, D. et al. Influence of postprandial hyperglycemic conditions on arterial stiffness in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 101, 1134–1143 (2016).
Carlson, O. et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 56, 1729–1734 (2007).
Clark, L. T. Alcohol-induced hypertension: mechanisms, complications, and clinical implications. J. Natl Med. Assoc. 77, 385–389 (1985).
Mayl, J. J. et al. Association of alcohol intake with hypertension in type 2 diabetes mellitus: the ACCORD Trial. J. Am. Heart Assoc. 9, e017334 (2020).
Howes, L. G. & Reid, J. L. The effects of alcohol on local, neural and humoral cardiovascular regulation. Clin. Sci. 71, 9–15 (1986).
Hwang, C.-L., Muchira, J., Hibner, B. A., Phillips, S. A. & Piano, M. R. Alcohol consumption: a new risk factor for arterial stiffness? Cardiovasc. Toxicol. 22, 236–245 (2022).
Tian, D. & Meng, J. Exercise for prevention and relief of cardiovascular disease: prognoses, mechanisms, and approaches. Oxid. Med. Cell. Longev. 2019, 3756750 (2019).
Shahraki, M. R., Mirshekari, H., Shahraki, A. R., Shahraki, E. & Naroi, M. Arterial blood pressure in female students before, during and after exercise. ARYA Atheroscler. 8, 12–15 (2012).
Coates, A. M., Joyner, M. J., Little, J. P., Jones, A. M. & Gibala, M. J. A perspective on high-intensity interval training for performance and health. Sport Med. 53, 85–96 (2023).
Mohammed, L. L. M. et al. Exercise-induced hypertension in healthy individuals and athletes: is it an alarming sign? Cureus 12, e11988 (2020).
MacDonald, J. R. Potential causes, mechanisms, and implications of post exercise hypotension. J. Hum. Hypertens. 16, 225–236 (2002).
Parkes, J. L., Slatin, S. L., Pardo, S. & Ginsberg, B. H. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 23, 1143–1148 (2000).
Madsen, J. L., Søndergaard, S. B. & Møller, S. Meal-induced changes in splanchnic blood flow and oxygen uptake in middle-aged healthy humans. Scand. J. Gastroenterol. 41, 87–92 (2006).
Jones, A. W. & Jönsson, K. A. Food-induced lowering of blood-ethanol profiles and increased rate of elimination immediately after a meal. J. Forensic Sci. 39, 1084–1093 (1994).
Richter, B. et al. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 10, CD012661 (2018).
Warren, B. et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 5, 34–42 (2017).
Huang, Y. et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 355, i5953 (2016).
Rooney, M. R. et al. Global prevalence of prediabetes. Diabetes Care 46, 1388–1394 (2023).
Hu, J.-R. et al. Effects of carbohydrate quality and amount on plasma lactate: results from the OmniCarb trial. BMJ Open Diabetes Res. Care 8, e001457 (2020).
Emhoff, C.-A.W. & Messonnier, L.A. Concepts of lactate metabolic clearance rate and lactate clamp for metabolic inquiry: a mini-review. Nutrients 15, 3213 (2023).
Rynders, C. A. et al. Effects of exercise intensity on postprandial improvement in glucose disposal and insulin sensitivity in prediabetic adults. J. Clin. Endocrinol. Metab. 99, 220–228 (2014).
Chai, Y. et al. Association of body mass index with risk of prediabetes in Chinese adults: a population-based cohort study. J. Diabetes Investig. 13, 1235–1244 (2022).
Shapiro, N. I. et al. Lactate as a predictor of mortality in patients with severe sepsis. Crit. Care Med. 37, 1678–1684 (2009).
Bishop, N. D. et al. The role of lactate in the assessment of the critically ill. J. Criti. Care 37, 110–115 (2017).
Bodenheimer, T. & Handley, M. A. Goal-setting for behavior change in diabetes: what’s the evidence? Diabetes Educ. 35, 775–782 (2009).
Fisher, L. et al. The role of self-monitoring in diabetes management: a review. Diabetes Spectrum 29, 129–135 (2016).
Tabák, A. G. et al. Prediabetes: a high-risk state for developing diabetes. Lancet 379, 2279–2290 (2012).
Zhang, Y. et al. Artificial intelligence in diabetes care: current applications and future directions. Diabetes Care 43, 141–148 (2020).
Lin, M. et al. A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects. Nat. Biotechnol. 42, 448–457 (2024).
Lv, J. et al. Printable elastomeric electrodes with sweat-enhanced conductivity for wearables. Sci. Adv. 7, eabg8433 (2021).
Yin, L. et al. A stretchable epidermal sweat sensing platform with an integrated printed battery and electrochromic display. Nat. Electron. 5, 694–705 (2022).
Kim, J. et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. 5, 1800880 (2018).
Kim, J. et al. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 1, 1011–1019 (2016).
Butman, S. M., Ewy, G. A., Standen, J. R., Kern, K. B. & Hahn, E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J. Am. Coll. Cardiol. 22, 968–974 (1993).
Baruch, M. C., Kalantari, K., Gerdt, D. W. & Adkins, C. M. Validation of the pulse decomposition analysis algorithm using central arterial blood pressure. Biomed. Eng. Online 13, 96 (2014).
Izzo, J. L. Pulse contour analysis and augmentation index: It’s time to move beyond cuff blood pressure measurement. Am. J. Hypertens. 18, 1S–2S (2005).
Chang, A.-Y. et al. Processing-scripts-for-monitoring-metabolites-and-cardiac-signals-in-diabetes. Zenodo https://doi.org/10.5281/zenodo.15476742 (2025).
Acknowledgements
This research is supported by the UCSD Center for Wearable Sensors (CWS). M.R. received support from the UC-MEXUS-CONAHCYT Doctoral Fellowship. We thank S. Suresh for assistance.
Author information
Authors and Affiliations
Contributions
A.-Y.C., M.L., L.Y., M.R., S.X. and J.W. conceived and designed the research. A.-Y.C., M.L., L.Y. and M.R. conducted the experiments. A.-Y.C., M.L., L.Y., M.R., S.D., R.L., Y.D., A.C., G.P., Z.L., H.L. and N.A. performed the experiments. A.-Y.C., M.L., L.Y. and M.R. analysed the data. A.-Y.C., M.L., L.Y., M.R., S.X. and J.W. wrote the manuscript with valuable feedback and assistance from other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Chi Hwan Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussions 1–10, reference, Figs. 1–32, Tables 1 and 2, and Videos 1–6.
Supplementary Video 1
The BLUE wristband platform was worn on the participants wrist during jumping rope.
Supplementary Video 2
The BLUE wristband platform was worn on the participants wrist while dribbling the basketball.
Supplementary Video 3
The BLUE wristband platform was worn on the participant’s wrist while doing push-ups.
Supplementary Video 4
The BLUE wristband platform on the participant’s wrist was worn in an inverted direction to enhance the microneedle array’s visibility and demonstrate finger contact with the ECG system.
Supplementary Video 5
Demonstrating the installation and disposal of the microneedle array: the microneedle array is assembled and disassembled to the wristband.
Supplementary Video 6
Skin penetration stimulation of the microneedle system.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, AY., Lin, M., Yin, L. et al. Integration of chemical and physical inputs for monitoring metabolites and cardiac signals in diabetes. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01439-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-025-01439-z