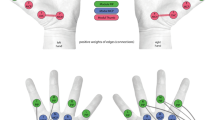
Abstract
Much of human memory takes the form of cognitive graphs that allow us to relate and generalize knowledge. The influence of structured memory in the motor system is less clear. Here we examine how structured memory representations influence action selection when responses are retrieved from newly learned, hierarchical visuomotor maps. Human participants (N = 182) learned visuomotor mappings with (or without) an imposed latent structure that linked visual stimulus features (for example, colour or shape) to intuitive motor distinctions, such as hands and pairs of fingers. In participants who learned structured visuomotor mappings, transitional response times indicated that retrieving the correct response from memory invoked the ‘traversal’ of a structured mental graph. Forced-response experiments revealed similar computations within individual trials. Moreover, graph-like representations persisted even after multiple days of practice with the visuomotor mappings. Our results point to direct links between internal computations over structured memory representations and the preparation of movements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available via GitHub at https://github.com/jetrach/StructuredActionPrepVMDM.
Code availability
The code used is available via GitHub at https://github.com/jetrach/StructuredActionPrepVMDM. The task code is available upon request. Please refer to the Methods for details on the software used in this project.
References
Trach, J. E., McKim, T. H. & Desrochers, T. M. Abstract sequential task control is facilitated by practice and embedded motor sequences. J. Exp. Psychol. Learn. Mem. Cogn. 47, 1638–1659 (2021).
Peer, M., Brunec, I. K., Newcombe, N. S. & Epstein, R. A. Structuring knowledge with cognitive maps and cognitive graphs. Trends Cogn. Sci. 25, 37–54 (2021).
Schapiro, A. C., Rogers, T. T., Cordova, N. I., Turk-Browne, N. B. & Botvinick, M. M. Neural representations of events arise from temporal community structure. Nat. Neurosci. 16, 486–492 (2013).
Schuck, N. W., Cai, M. B., Wilson, R. C. & Niv, Y. Human orbitofrontal cortex represents a cognitive map of state space. Neuron 91, 1402–1412 (2016).
Kahn, A. E., Karuza, E. A., Vettel, J. M. & Bassett, D. S. Network constraints on learnability of probabilistic motor sequences. Nat. Hum. Behav. 2, 936–947 (2018).
Rosenbaum, D. A., Kenny, S. B. & Derr, M. A. Hierarchical control of rapid movement sequences. J. Exp. Psychol. Hum. Percept. Perform. 9, 86–102 (1983).
Cisek, P. & Pastor-Bernier, A. On the challenges and mechanisms of embodied decisions. Phil. Trans. R. Soc. B 369, 20130479 (2014).
Gallivan, J. P., Chapman, C. S., Wolpert, D. M. & Flanagan, J. R. Decision-making in sensorimotor control. Nat. Rev. Neurosci. 19, 519–534 (2018).
Gold, J. I. & Shadlen, M. N. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 (2007).
Selen, L. P. J., Shadlen, M. N. & Wolpert, D. M. Deliberation in the motor system: reflex gains track evolving evidence leading to a decision. J. Neurosci. 32, 2276–2286 (2012).
Shadlen, M. N. & Kiani, R. Decision making as a window on cognition. Neuron 80, 791–806 (2013).
Song, J.-H. & Nakayama, K. Target selection in visual search as revealed by movement trajectories. Vis. Res. 48, 853–861 (2008).
Song, J.-H. & Nakayama, K. Numeric comparison in a visually-guided manual reaching task. Cognition 106, 994–1003 (2008).
Song, J.-H. & Nakayama, K. Hidden cognitive states revealed in choice reaching tasks. Trends Cogn. Sci. 13, 360–366 (2009).
Spivey, M. J. & Dale, R. Continuous dynamics in real-time cognition. Curr. Dir. Psychol. Sci. 15, 207–211 (2006).
Spivey, M. J., Grosjean, M. & Knoblich, G. Continuous attraction toward phonological competitors. Proc. Natl Acad. Sci. USA 102, 10393–10398 (2005).
Ghez, C. et al. Discrete and continuous planning of hand movements and isometric force trajectories. Exp. Brain Res. 115, 217–233 (1997).
Hardwick, R. M., Forrence, A. D., Krakauer, J. W. & Haith, A. M. Time-dependent competition between goal-directed and habitual response preparation. Nat. Hum. Behav. 3, 1252–1262 (2019).
Mcdougle, S. D. & Taylor, J. A. Dissociable cognitive strategies for sensorimotor learning. Nat. Commun. 10, 40 (2019).
Constantinescu, A. O., O’Reilly, J. X. & Behrens, T. E. J. Organizing conceptual knowledge in humans with a gridlike code. Science 352, 1464–1467 (2016).
Musslick, S. & Bizyaeva, A. Examining cognitive flexibility and stability through the lens of dynamical systems. Curr. Opin. Behav. Sci. 57, 101375 (2024).
Collins, A. G. E. & Frank, M. J. Motor demands constrain cognitive rule structures. PLoS Comput. Biol. https://doi.org/10.1371/journal.pcbi.1004785 (2016).
Dykstra, T., Smith, D. M., Schumacher, E. H. & Hazeltine, E. Measuring task structure with transitional response times: task representations are more than task sets. Psychon. Bull. Rev. https://doi.org/10.3758/s13423-021-02035-3 (2022).
Reitman, J. S. & Rueter, H. H. Organization revealed by recall orders and confirmed by pauses. Cogn. Psychol. 12, 554–581 (1980).
Maechler, M. R., Rousseeuw, P., Struyf, A., Hubert, M. & Hornik, K. cluster: Cluster analysis basics and extensions. R package version 2.1.3 (2022).
Kassambara, A. & Mundt, F. factoextra: Extract and visualize the results of multivariate data analyses. R package version 1.0.7 (2020).
Brown, T. I. et al. Prospective representation of navigational goals in the human hippocampus. Science 352, 1323–1326 (2016).
Rmus, M., Ritz, H., Hunter, L. E., Bornstein, A. M. & Shenhav, A. Humans can navigate complex graph structures acquired during latent learning. Cognition 225, 105103 (2022).
Tavares, R. M. et al. A map for social navigation in the human brain. Neuron 87, 231–243 (2015).
Karuza, E. A., Kahn, A. E., Thompson-Schill, S. L. & Bassett, D. S. Process reveals structure: how a network is traversed mediates expectations about its architecture. Sci. Rep. 7, 12733 (2017).
Rosenbaum, D. A. Human movement initiation: specification of arm, direction, and extent. J. Exp. Psychol. Gen. 109, 444–474 (1980).
Jaffe, P. I., Poldrack, R. A., Schafer, R. J. & Bissett, P. G. Modelling human behaviour in cognitive tasks with latent dynamical systems. Nat. Hum. Behav. https://doi.org/10.1038/s41562-022-01510-8 (2023).
Balaguer, J., Spiers, H., Hassabis, D. & Summerfield, C. Neural mechanisms of hierarchical planning in a virtual subway network. Neuron 90, 893–903 (2016).
Ekstrom, A. D. & Ranganath, C. Space, time, and episodic memory: the hippocampus is all over the cognitive map. Hippocampus 28, 680–687 (2018).
Theves, S., Neville, D. A., Fernández, G. & Doeller, C. F. Learning and representation of hierarchical concepts in hippocampus and prefrontal cortex. J. Neurosci. 41, 7675–7686 (2021).
Whittington, J. C. R. et al. The Tolman–Eichenbaum machine: unifying space and relational memory through generalization in the hippocampal formation. Cell 183, 1249–1263.e23 (2020).
Wu, C. M., Schulz, E., Garvert, M. M., Meder, B. & Schuck, N. W. Similarities and differences in spatial and non-spatial cognitive maps. PLoS Comput. Biol. 16, e1008149 (2020).
Zhou, J. et al. Complementary task structure representations in hippocampus and orbitofrontal cortex during an odor sequence task. Curr. Biol. 29, 3402–3409.e3 (2019).
Burk, D., Ingram, J. N., Franklin, D. W., Shadlen, M. N. & Wolpert, D. M. Motor effort alters changes of mind in sensorimotor decision making. PLoS ONE 9, e92681 (2014).
Cisek, P. & Kalaska, J. F. Simultaneous encoding of multiple potential reach directions in dorsal premotor cortex. J. Neurophysiol. 87, 1149–1154 (2002).
Hagura, N., Haggard, P. & Diedrichsen, J. Perceptual decisions are biased by the cost to act. eLife 6, e18422 (2017).
Moher, J. & Song, J.-H. Perceptual decision processes flexibly adapt to avoid change-of-mind motor costs. J. Vis. 14, 1 (2014).
Selen, L. P. J., Corneil, B. D. & Medendorp, W. P. Single-trial dynamics of competing reach plans in the human motor periphery. J. Neurosci. 43, 2782–2793 (2023).
Thura, D. & Cisek, P. Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron 81, 1401–1416 (2014).
Wong, A. L. & Haith, A. M. Motor planning flexibly optimizes performance under uncertainty about task goals. Nat. Commun. 8, 14624 (2017).
Freeman, J. B., Nakayama, K. & Ambady, N. Finger in flight reveals parallel categorization across multiple social dimensions. Soc. Cogn. 31, 792–805 (2013).
Magnuson, J. S. Moving hand reveals dynamics of thought. Proc. Natl Acad. Sci. USA 102, 9995–9996 (2005).
Alhussein, L. & Smith, M. A. Motor planning under uncertainty. eLife 10, e67019 (2021).
Haith, A. M., Huberdeau, D. M. & Krakauer, J. W. Hedging your bets: intermediate movements as optimal behavior in the context of an incomplete decision. PLoS Comput. Biol. 11, e1004171 (2015).
Miller, J. Discrete versus continuous stage models of human information processing: in search of partial output. J. Exp. Psychol. Hum. Percept. Perform. 8, 273–296 (1982).
Cellier, D., Petersen, I. T. & Hwang, K. Dynamics of hierarchical task representations. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.0233-22.2022 (2022).
Ranti, C., Chatham, C. H. & Badre, D. Parallel temporal dynamics in hierarchical cognitive control. Cognition 142, 205–229 (2015).
Badre, D. & D’Esposito, M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 10, 659–669 (2009).
Badre, D. & Nee, D. E. Frontal cortex and the hierarchical control of behavior. Trends Cogn. Sci. 22, 170–188 (2018).
Logan, G. D. Toward an instance theory of automatization. Psychol. Rev. 95, 492–527 (1988).
Logan, G. D. Automatic control: how experts act without thinking. Psychol. Rev. 125, 453–485 (2018).
Broadbent, D. E. & Broadbent, M. H. P. From detection to identification: response to multiple targets in rapid serial visual presentation. Percept. Psychophys. 42, 105–113 (1987).
McLean, J. P., Broadbent, D. E. & Broadbent, M. H. P. Combining attributes in rapid serial visual presentation tasks. Q. J. Exp. Psychol. https://doi.org/10.1080/14640748308402123 (1983).
Treisman, A. Focused attention in the perception and retrieval of multidimensional stimuli. Percept. Psychophys. 22, 1–11 (1977).
Behrens, T. E. J. et al. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018).
Chrastil, E. R. & Warren, W. H. From cognitive maps to cognitive graphs. PLoS ONE 9, e112544 (2014).
de Leeuw, J. R., Gilbert, R. A. & Luchterhandt, B. jsPsych: Enabling an open-source collaborative ecosystem of behavioral experiments. J. Open Source Softw. 8, 5351 (2023).
Eaton, J. W. GNU Octave and reproducible research. J. Process Control 22, 1433–1438 (2012).
Brainard, D. H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
R Core Team. R: A language and environment for statistical computing (R Foundation for Statistical Computing, 2022); https://www.R-project.org/
MATLAB version 9.13.0 (R2022a) (The MathWorks Inc., 2022); https://www.mathworks.com
Torchiano, M. effsize: Efficient effect size computation. R package version 0.8.1 (2020).
McDougle, S. D. Post-error slowing during instrumental learning is shaped by working memory-based choice strategies. Neuroscience 486, 37–45 (2022).
Acknowledgements
We thank A. Forrence and T. Adanri in particular for their help with these projects. In addition, we acknowledge M. Ingram, J. Burde, K. Chou, S. Hu, L. Pandolpho and O. Pilkinton for essential support in collecting data. We also thank T. Desrochers and J. Taylor for feedback on early drafts of the manuscript, and the members of the ACT lab and Turk-Browne Lab at Yale University for productive conversations about this work throughout. J.E.T. is supported by the NSF GRFP. S.D.M. is supported by NIH grant no. R01 NS132926.
Author information
Authors and Affiliations
Contributions
J.E.T. and S.D.M. designed the paradigm. J.E.T. collected the data. J.E.T. and S.D.M. analysed the data. J.E.T. and S.D.M. prepared the figures and drafted, edited and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Aymar de Rugy, Elliot Hazeltine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Table 1 and Results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trach, J.E., McDougle, S.D. Mental graphs structure the storage and retrieval of visuomotor associations. Nat Hum Behav (2025). https://doi.org/10.1038/s41562-025-02217-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41562-025-02217-2