Abstract
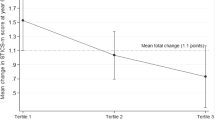
A healthy diet has been associated with a reduced risk of dementia. Here we devised a Machine learning-assisted Optimizing Dietary intERvention against demeNtia risk (MODERN) diet based on data from 185,012 UK Biobank participants, 1,987 of whom developed all-cause dementia over 10 years. We first identified 25 food groups associated with dementia in a food-wide association analysis. Second, we ranked their importance using machine learning and prioritized eight groups (for example, green leafy vegetables, berries and citrus fruits). Finally, we established and externally validated a MODERN score (0–7), which showed stronger associations with lower risk of dementia-related outcomes (hazard ratio comparing highest versus lowest tertiles: 0.64, 95% CI: 0.43–0.93) than the a priori-defined MIND diet (0.75, 0.61–0.92). Across 63 health-related outcomes, the MODERN diet showed particularly significant associations with mental/behavioural disorders. Multimodal neuroimaging, metabolomics, inflammation and proteomics analyses revealed potential pathways and further support the potential of MODERN diet for dementia prevention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main dataset supporting the conclusions of this article is available in the UK Biobank (UKB) repository (https://www.ukbiobank.ac.uk/). The disease and death outcomes in UKB can be obtained from the following restricted access national healthcare databases: the Hospital Episode Statistics (https://digital.nhs.uk/services/hospital-episode-statistics), the Scottish Morbidity Records (https://www.ndc.scot.nhs.uk/National-Datasets/data-dictionary-smr01/), the National Health Service Information Center (https://digital.nhs.uk) and Central Register Scotland (https://www.nrscotland.gov.uk). This study utilized the UKB Resource under application number 19542. The Health and Retirement Study dataset is publicly available through its website (https://hrsdata.isr.umich.edu/data-products/2013-health-care-and-nutrition-study-hcns). The data from the Framingham Heart Study Offspring Cohort can be applied for at http://www.framinghamheartstudy.org/researchers/index.php. This study utilized the FOS data under application number 11068. The National Health and Nutrition Examination Survey data are publicly available through the CDC/NCHS website (https://www.cdc.gov/nchs/nhanes/). The summary statistics of the AD GWAS can be accessed at https://gwas.mrcieu.ac.uk/datasets/ieu-b-2/.
Code availability
The analysis programmes can be accessed on GitHub at https://github.com/Happychrischen/Diet_ML_Dementia (ref. 70).
References
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Chen, H. et al. Association of the Mediterranean Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) diet with the risk of dementia. JAMA Psychiatry 80, 630–638 (2023).
Zhang, Y. et al. Identifying modifiable factors and their joint effect on dementia risk in the UK Biobank. Nat. Hum. Behav. 7, 1185–1195 (2023).
Morris, M. C. et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 11, 1015–1022 (2015).
Morris, M. C. et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 11, 1007–1014 (2015).
van den Brink, A. C., Brouwer-Brolsma, E. M., Berendsen, A. A. M. & van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease—a review. Adv. Nutr. 10, 1040–1065 (2019).
Chen, H. et al. Associations of the Mediterranean‐DASH Intervention for Neurodegenerative Delay diet with brain structural markers and their changes. Alzheimers Dement. 20, 1190–1200 (2024).
Samuelsson, J. et al. Associations between dietary patterns and dementia‐related neuroimaging markers. Alzheimers Dement. 19, 4629–4640 (2023).
Agarwal, P. et al. Association of Mediterranean-DASH Intervention for Neurodegenerative Delay and Mediterranean diets with Alzheimer disease pathology. Neurology 100, e2259–e2268 (2023).
Cornelis, M. C., Agarwal, P., Holland, T. M. & van Dam, R. M. MIND dietary pattern and its association with cognition and incident dementia in the UK Biobank. Nutrients 15, 32 (2022).
Vu, T. H. T. et al. Adherence to MIND diet, genetic susceptibility, and incident dementia in three US cohorts. Nutrients 14, 2759 (2022).
de Crom, T. O. E., Mooldijk, S. S., Ikram, M. K., Ikram, M. A. & Voortman, T. MIND diet and the risk of dementia: a population-based study. Alzheimers Res. Ther. 14, 8 (2022).
Barnes, L. L. et al. Trial of the MIND diet for prevention of cognitive decline in older persons. N. Engl. J. Med. 389, 602–611 (2023).
Piernas, C. et al. Describing a new food group classification system for UK Biobank: analysis of food groups and sources of macro- and micronutrients in 208,200 participants. Eur. J. Nutr. 60, 2879–2890 (2021).
Zhang, S., Tomata, Y., Sugiyama, K., Sugawara, Y. & Tsuji, I. Citrus consumption and incident dementia in elderly Japanese: the Ohsaki Cohort 2006 Study. Br. J. Nutr. 117, 1174–1180 (2017).
Zafra‐Stone, S. et al. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 51, 675–683 (2007).
Nakajima, A. et al. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res. 250, 351–360 (2013).
Muhammad, T., Ikram, M., Ullah, R., Rehman, S. & Kim, M. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 11, 648 (2019).
Zhang, R. et al. Associations of dietary patterns with brain health from behavioral, neuroimaging, biochemical and genetic analyses. Nat. Mental Health 2, 535–552 (2024).
Jeon, K. H. et al. Changes in alcohol consumption and risk of dementia in a nationwide cohort in South Korea. JAMA Netw. Open 6, e2254771 (2023).
Rao, R. T. Methodological difficulties of studying alcohol consumption and dementia. BMJ 362, k3894 (2018).
Zhang, Y. et al. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 103, 330–340 (2016).
Wan, Y. et al. Association between changes in carbohydrate intake and long term weight changes: prospective cohort study. BMJ 382, e073939 (2023).
Ayoob, K. T. Carbohydrate confusion and dietary patterns: unintended public health consequences of ‘food swapping’. Front. Nutr. 10, 1266308 (2023).
Drewnowski, A., Maillot, M. & Vieux, F. Multiple metrics of carbohydrate quality place starchy vegetables alongside non-starchy vegetables, legumes, and whole fruit. Front. Nutr. 9, 867378 (2022).
Ylilauri, M. P. et al. Association of dietary cholesterol and egg intakes with the risk of incident dementia or Alzheimer disease: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 105, 476–484 (2017).
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (National Academies Press, 1998).
Vishwanathan, R., Kuchan, M. J., Sen, S. & Johnson, E. J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 59, 659–665 (2014).
Chen, Y. et al. Associations of sugar-sweetened, artificially sweetened, and naturally sweet juices with Alzheimer’s disease: a prospective cohort study. GeroScience 46, 1229–1240 (2023).
Liu, H. et al. Meta-analysis of sugar-sweetened beverage intake and the risk of cognitive disorders. J. Affect. Disord. 313, 177–185 (2022).
Chen, H. et al. Sugary beverages and genetic risk in relation to brain structure and incident dementia: a prospective cohort study. Am. J. Clin. Nutr. 117, 672–680 (2023).
Scheltens, P. et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021).
Schliebs, R. & Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 221, 555–563 (2011).
O’Brien, J. T. Clinical significance of white matter changes. Am. J. Geriatr. Psychiatry 22, 133–137 (2014).
Roseborough, A., Ramirez, J., Black, S. E. & Edwards, J. D. Associations between amyloid β and white matter hyperintensities: a systematic review. Alzheimers Dement. 13, 1154–1167 (2017).
Peng, M. et al. Dietary inflammatory index, genetic susceptibility and risk of incident dementia: a prospective cohort study from UK Biobank. J. Neurol. 271, 1286–1296 (2024).
Chen, X., Maguire, B., Brodaty, H. & O’Leary, F. Dietary patterns and cognitive health in older adults: a systematic review. J. Alzheimers Dis. 67, 583–619 (2019).
Dyall, S. C. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 7, 52 (2015).
Chen, H. et al. Circulating metabolomic profile of the MIND diet and its relation to cognition in middle-aged and older adults. iMetaOmics 2, e61 (2025).
McGrattan, A. M. et al. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 8, 53–65 (2019).
Pereira, J. B. et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain 144, 3505–3516 (2021).
Benedet, A. L. et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 78, 1471–1483 (2021).
Pontecorvo, M. J. et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 79, 1250–1259 (2022).
Huber, H. et al. Biomarkers of Alzheimer’s disease and neurodegeneration in dried blood spots—A new collection method for remote settings. Alzheimers Dement. 20, 2340–2352 (2024).
Guo, Y. et al. Plasma proteomic profiles predict future dementia in healthy adults. Nat. Aging 4, 247–260 (2024).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Liu, B. et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 14, 1998–2005 (2011).
Bradbury, K. E., Young, H. J., Guo, W. & Key, T. J. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 7, e6 (2018).
Perez-Cornago, A. et al. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur. J. Nutr. 60, 4019–4030 (2021).
Greenwood, D. C. et al. Validation of the Oxford WebQ online 24-hour dietary questionnaire using biomarkers. Am. J. Epidemiol. 188, 1858–1867 (2019).
Liu, W. et al. Association of biological age with health outcomes and its modifiable factors. Aging Cell 22, e13995 (2023).
Cheesman, R. et al. Familial influences on neuroticism and education in the UK Biobank. Behav. Genet. 50, 84–93 (2020).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Bond, J., Townsend, P., Phillimore, P. & Beattie, A. Health deprivation: inequality and the North, Croom Helm, Beckenham, 1987. J. Soc. Policy 18, 291–293 (1989).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395 (2003).
Ke, G. et al. LightGBM: a highly efficient gradient boosting decision tree. In Proc. 31st Conference on Neural Information Processing Systems (eds Guyon, I. et al.) 3147–3155 (NIPS, 2017).
Sonnega, A. et al. Cohort Profile: the Health and Retirement Study (HRS). Int. J. Epidemiol. 43, 576–585 (2014).
Crimmins, E. M., Kim, J. K., Langa, K. M. & Weir, D. R. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J. Gerontol. B 66, i162–i171 (2011).
NHANES Survey Methods and Analytic Guidelines (CDC, NCHS, year); https://wwwn.cdc.gov/Nchs/Nhanes/AnalyticGuidelines.aspx
Ahluwalia, N., Dwyer, J., Terry, A., Moshfegh, A. & Johnson, C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv. Nutr. 7, 121–134 (2016).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458 (2013).
Lourida, I. et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA 322, 430–437 (2019).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536 (2016).
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166, 400–424 (2018).
Wik, L. et al. Proximity extension assay in combination with next-generation sequencing for high-throughput proteome-wide analysis. Mol. Cell. Proteomics 20, 100168 (2021).
Hu, F. B. et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 69, 243–249 (1999).
Raghunathan, T., Lepkowski, J., Hoewyk, J. V. & Solenberger, P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv. Methodol. 27, 85–95 (2001).
Szklarczyk, D. et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Alfaro-Almagro, F. et al. Confound modelling in UK Biobank brain imaging. Neuroimage 224, 117002 (2021).
Chen, S. J. Diet-ML-Dementia. Zenodo https://doi.org/10.5281/zenodo.15671234 (2025).
Acknowledgements
We thank all the participants and professionals contributing to the UK Biobank and the Health and Retirement Study. This study was supported by grants from the Science and Technology Innovation 2030 Major Projects (2022ZD0211600 to J.-T.Y.), the National Natural Science Foundation of China (82071201, 82271471 and 92249305 to J.-T.Y.; 82071997 and 82472055 to W.C.; 8210120183 to C.Y.; 82402381 and 82471940 to J.Y.), the Shanghai Municipal Science and Technology Major Project (2023SHZDZX02 to J.-T.Y., 2018SHZDZX01 to J.-F.F.), the National Nutrition Science Research Grant (CNS-NNSRG2021-61 to C.Y.), the Zhejiang University Global Partnership Fund, Shanghai Pujiang Talent Program (23PJD006 to J.Y.), a Research Start-up Fund of Huashan Hospital (2022QD002 to J.-T.Y.), the Program of Shanghai Academic Research Leader (23XD1420400 to J.-T.Y.), the Excellence 2025 Talent Cultivation Program at Fudan University (3030277001 to J.-T.Y.), Shanghai Talent Development Funding for The Project (2019074), Shanghai Rising-Star Program (21QA1408700 to W.C.), 111 Project (B18015 to J.-F.F.), the National Postdoctoral Program for Innovative Talents (BX20230087 to S.-D.C.), the China Postdoctoral Science Foundation (2023M740672 to S.-D.C.), and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of the Ministry of Education, and Shanghai Center for Brain Science and Brain-Inspired Technology, Fudan University. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
J.-T.Y. and C.Y. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. J.-T.Y. conceptualized and designed the project. All authors acquired, analysed or interpreted data. S.-J.C., S.-D.C., H.C., J.Y., C.Y. and J.-T.Y. drafted the paper. S.-J.C., H.C., J.Y., S.-D.C., L.H., X.G., W.C., C.Y. and J.-T.Y. critically revised the paper for important intellectual content. S.-J.C., J.Y., H.C., Y.F., W.Z. and W.C. performed statistical analysis. J.Y., S.-D.C., J.-F.F., W.C., C.Y. and J.-T.Y. obtained funding. J.-F.F., W.C., C.Y. and J.-T.Y. provided administrative, technical or material support. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Rob van Dam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The associations between the components of the MODERN diet and ACD risk in 185,012 UKB participants.
a. The Kaplan-Meier curves of ACD-free survival in participants receiving 0 vs. 1 point for each food component. Shaded areas showed 95% confidence intervals. b. The HR for incident ACD comparing 1 vs. 0 point was estimated using the Cox proportional hazard regression model. A two-sided Wald test was performed to assess statistical significance. The significant results (P value < 0.05) were denoted in red in the forest plot. The analyses were adjusted for total energy intake, age, sex, ethnicity, TDI, educational attainment, smoking status, physical activity, BMI, ApoE-ε4 gene, and history of hypertension, diabetes, cerebrovascular disease, and other CVDs. ACD: all-cause dementia; TDI: Townsend deprivation index; BMI: body mass index; CVDs: cardiovascular diseases.
Extended Data Fig. 2 The associations between the MODERN diet and ACD risk in 185,012 UKB participants.
a. The associations of MODERN and MIND diet with incident ACD using the Cox proportional hazard regression model. b. The nonlinear associations between MODERN and MIND diet score and incident dementia using the RCS. A two-sided Wald test was performed to assess statistical significance. The significant results (P value < 0.05) were denoted in red in the forest plot. The dashed lines showed 95% confidence intervals. The analyses were adjusted for total energy intake, age, sex, ethnicity, TDI, educational attainment, smoking status, physical activity, BMI, ApoE-ε4 gene, and history of hypertension, diabetes, cerebrovascular disease, and other CVDs. MIND: Mediterranean-DASH Intervention for Neurodegenerative Delay; ACD: all-cause dementia; RCS: restricted cubic spline; TDI: Townsend deprivation index; BMI: body mass index; CVDs: cardiovascular diseases.
Extended Data Fig. 3 The inclusion and exclusion criteria of participants in external cohorts.
HRS: Health and Retirement Study. FOS: Framingham Heart Study Offspring Cohort. NHANES: National Health and Nutrition Examination Survey.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Supplementary Table 1
Supplementary Tables 1–23.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, SJ., Chen, H., You, J. et al. Machine learning-assisted optimization of dietary intervention against dementia risk. Nat Hum Behav (2025). https://doi.org/10.1038/s41562-025-02255-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41562-025-02255-w