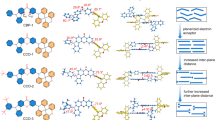
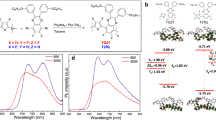
Abstract
Blue thermally activated delayed fluorescent emitters are promising for the next generation of organic light-emitting diodes, yet their performance still cannot meet the requirements for commercialization. Here we establish a design rule for highly efficient and stable thermally activated delayed fluorescent emitters by introducing an auxiliary acceptor that could delocalize electron distributions, enhancing molecular stability in both the negative polaron and triplet excited state, while also accelerating triplet-to-singlet up-conversion and singlet radiative processes simultaneously. Proof-of-concept thermally activated delayed fluorescent compounds, based on a multi-carbazole-benzonitrile structure, exhibit near-unity photoluminescent quantum yields, short-lived delays and improved photoluminescent and electroluminescent stabilities. A deep-blue organic light-emitting diode using one of these molecules as a sensitizer for a multi-resonance emitter achieves a remarkable time to 95% of initial luminance of 221 h at an initial luminance of 1,000 cd m−2, a maximum external quantum efficiency of 30.8% and Commission Internationale de l’Eclairage coordinates of (0.14, 0.17).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the CCDC under deposition numbers 2232729, 2232730 and 2232731. These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Sun, J. et al. Exceptionally stable blue phosphorescent organic light-emitting diodes. Nat. Photon. 16, 212–218 (2022).
Baldo, M. A. et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Ai, X. et al. Efficient radical-based light-emitting diodes with doublet emission. Nature 563, 536–540 (2018).
Ma, Y. & Yang, B. Progress in next-generation organic electroluminescent materials: material design beyond exciton statistics. Sci. Sin. Chim. 43, 1457–1467 (2013).
Endo, A. et al. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 98, 083302 (2011).
Goushi, K., Yoshida, K., Sato, K. & Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photon. 6, 253–258 (2012).
Masui, K., Nakanotani, H. & Adachi, C. Analysis of exciton annihilation in high-efficiency sky-blue organic light-emitting diodes with thermally activated delayed fluorescence. Org. Electron. 14, 2721–2726 (2013).
Chan, C.-Y. et al. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photon. 15, 203–207 (2021).
Zhang, D. et al. Efficient and stable deep‐blue fluorescent organic light‐emitting diodes employing a sensitizer with fast triplet upconversion. Adv. Mater. 32, 1908355 (2020).
Jeon, S. O. et al. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat. Photon. 15, 208–215 (2021).
Kim, E. et al. Highly efficient and stable deep-blue organic light-emitting diode using phosphor-sensitized thermally activated delayed fluorescence. Sci. Adv. 8, eabq1641 (2022).
Mamada, M. et al. Highly efficient deep‐blue organic light‐emitting diodes based on rational molecular design and device engineering. Adv. Funct. Mater. 32, 2204352 (2022).
Tanaka, M., Noda, H., Nakanotani, H. & Adachi, C. Effect of carrier balance on device degradation of organic light‐emitting diodes based on thermally activated delayed fluorescence emitters. Adv. Electron. Mater. 5, 1800708 (2019).
Tanaka, M., Nagata, R., Nakanotani, H. & Adachi, C. Understanding degradation of organic light-emitting diodes from magnetic field effects. Commun. Mater. 1, 18 (2020).
Hasan, M. et al. Probing polaron-induced exciton quenching in TADF based organic light-emitting diodes. Nat. Commun. 13, 254 (2022).
Zhang, D., Cai, M., Zhang, Y., Zhang, D. & Duan, L. Sterically shielded blue thermally activated delayed fluorescence emitters with improved efficiency and stability. Mater. Horiz. 3, 145–151 (2016).
Noda, H. et al. Critical role of intermediate electronic states for spin-flip processes in charge-transfer-type organic molecules with multiple donors and acceptors. Nat. Mater. 18, 1084–1090 (2019).
Cui, L.-S. et al. Fast spin-flip enables efficient and stable organic electroluminescence from charge-transfer states. Nat. Photon. 14, 636–642 (2020).
Nakanotani, H. et al. High-efficiency organic light-emitting diodes with fluorescent emitters. Nat. Commun. 5, 4016 (2014).
Zhang, D. et al. High-efficiency fluorescent organic light-emitting devices using sensitizing hosts with a small singlet–triplet exchange energy. Adv. Mater. 26, 5050–5055 (2014).
Huang, T. et al. Enhancing the efficiency and stability of blue thermally activated delayed fluorescence emitters by perdeuteration. Nat. Photon. 18, 516–523 (2024).
Nagamura, N. et al. A multifunctional hole-transporter for high-performance TADF OLEDs and clarification of factors governing the transport property by multiscale simulation. J. Mater. Chem. C 10, 8694–8701 (2022).
Liu, T. et al. Zero–zero energy-dominated degradation in blue organic light-emitting diodes employing thermally activated delayed fluorescence. ACS Appl. Mater. Interfaces 14, 22332–22340 (2022).
Ha, T. H., Bin, J.-K. & Lee, C. W. Phenylpyridine and carbazole based host materials for highly efficient blue TADF OLEDs. Org. Electron. 102, 106450 (2022).
Ahn, D. H. et al. Rigid oxygen‐bridged boron‐based blue thermally activated delayed fluorescence emitter for organic light‐emitting diode: approach towards satisfying high efficiency and long lifetime together. Adv. Opt. Mater. 8, 2000102 (2020).
Byeon, S. Y., Han, S. H. & Lee, J. Y. Negative polaron‐stabilizing host for improved operational lifetime in blue phosphorescent organic light‐emitting diodes. Adv. Opt. Mater. 5, 1700387 (2017).
Wang, D., Cheng, C., Tsuboi, T. & Zhang, Q. Degradation mechanisms in blue organic light-emitting diodes. CCS Chem. 2, 1278–1296 (2020).
Wang, R., Meng, Q.-Y., Wang, Y.-L. & Qiao, J. Negative charge management to make fragile bonds less fragile toward electrons for robust organic optoelectronic materials. CCS Chem. 4, 331–343 (2022).
Lee, H. J., Lee, H. L., Han, S. H. & Lee, J. Y. Novel secondary acceptor based molecular design for superb lifetime in thermally activated delayed fluorescent organic light-emitting diodes through high bond energy and fast up-conversion. Chem. Eng. J. 427, 130988 (2022).
Ihn, S. et al. An alternative host material for long‐lifespan blue organic light‐emitting diodes using thermally activated delayed fluorescence. Adv. Sci. 4, 1600502 (2017).
Jeon, S. K., Lee, H. L., Yook, K. S. & Lee, J. Y. Recent progress of the lifetime of organic light‐emitting diodes based on thermally activated delayed fluorescent material. Adv. Mater. 31, 1803524 (2019).
Noda, H., Nakanotani, H. & Adachi, C. Excited state engineering for efficient reverse intersystem crossing. Sci. Adv. 4, eaao6910 (2018).
Chan, C.-Y., Tanaka, M., Nakanotani, H. & Adachi, C. Efficient and stable sky-blue delayed fluorescence organic light-emitting diodes with CIEy below 0.4. Nat. Commun. 9, 5036 (2018).
Huang, Y. et al. Electronic structures of interfacial states formed at polymeric semiconductor heterojunctions. Nat. Mater. 7, 483–489 (2008).
De Sio, A. et al. Tracking the coherent generation of polaron pairs in conjugated polymers. Nat. Commun. 7, 13742 (2016).
Zheng, X. et al. Expanding the hole delocalization range in excited molecules for stable organic light-emitting diodes employing thermally activated delayed fluorescence. J. Mater. Chem. C 8, 10021–10030 (2020).
Scholz, S., Walzer, K. & Leo, K. Analysis of complete organic semiconductor devices by laser desorption/ionization time-of-flight mass spectrometry. Adv. Funct. Mater. 18, 2541–2547 (2008).
Schmidbauer, S., Hohenleutner, A. & König, B. Chemical degradation in organic light-emitting devices: mechanisms and implications for the design of new materials. Adv. Mater. 25, 2114–2129 (2013).
Ryoo, C. H. et al. Systematic substituent control in blue thermally activated delayed fluorescence (TADF) emitters: unraveling the role of direct intersystem crossing between the same charge‐transfer states. Adv. Opt. Mater. 10, 2201622 (2022).
Kim, S.-Y. et al. Organic light-emitting diodes with 30% external quantum efficiency based on a horizontally oriented emitter. Adv. Funct. Mater. 23, 3896–3900 (2013).
Song, J., Lee, H., Jeong, E. G., Choi, K. C. & Yoo, S. Organic light‐emitting diodes: pushing toward the limits and beyond. Adv. Mater. 32, 1907539 (2020).
Chen, Y. et al. Approaching nearly 40% external quantum efficiency in organic light emitting diodes utilizing a green thermally activated delayed fluorescence emitter with an extended linear donor–acceptor–donor structure. Adv. Mater. 33, 2103293 (2021).
Hong, X. et al. TADF molecules with π-extended acceptors for simplified high-efficiency blue and white organic light-emitting diodes. Chem 8, 1705–1719 (2022).
Freidzon et al. Predicting the operational stability of phosphorescent OLED host molecules from first principles: a case study. J. Phys. Chem. C 121, 22422–22433 (2017).
Murawski, C., Leo, K. & Gather, M. C. Efficiency roll‐off in organic light‐emitting diodes. Adv. Mater. 25, 6801–6827 (2013).
Zhang, K. et al. Carbazole‐decorated organoboron emitters with low‐lying HOMO levels for solution‐processed narrowband blue hyperfluorescence OLED devices. Angew. Chem. Int. Ed. 62, e202313084 (2023).
Stavrou, K., Franca, L. G., Danos, A. & Monkman, A. P. Key requirements for ultraefficient sensitization in hyperfluorescence organic light-emitting diodes. Nat. Photon. 18, 554–561 (2024).
Kyulux reports rapid progress in emitter performance, is on track for commercial adoption in 2023. Kyulux https://www.kyulux.com/kyulux-reports-rapid-progress-in-emitter-performance-is-on-track-for-commercial-adoption-in-2023/ (2023).
Frisch, M. J. et al. Gaussian 16, revision B.01 (Gaussian, 2016).
Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2, 73–78 (2012).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Liu, Z., Lu, T. & Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: electronic structure, electronic spectrum, and optical nonlinearity. Carbon 165, 461–467 (2020).
Jung, Y. H. et al. Modified t-butyl in tetradentate platinum (II) complexes enables exceptional lifetime for blue-phosphorescent organic light-emitting diodes. Nat. Commun. 15, 2977 (2024).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (grant numbers 52222308 to D.Z. and 22135004 to L.D.), the National Key Research and Development Program (numbers 2022YFB3603002 to T.H. and 2023YFE0203300 to L.D.) and the Guangdong Major Project of Basic and Applied Basic Research (grant number 2019B030302009 to L.D.). We thank J. Qiao and Q. Meng from Tsinghua University for their assistance in calculating BDE values and providing valuable suggestions. We also extend our gratitude to H. Zhong, X. Wu and M. Yang from Beijing Institute of Technology for their support with photoelectrical ageing measurements. Additionally, we thank L. Wang and S. Wang from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, for providing the multi-resonance TADF material t-BuCz-DABNA.
Author information
Authors and Affiliations
Contributions
L.D. and D.Z. conceived and supervised this work. D.Z. proposed the molecular design concept and designed the experiments. T.H. carried out the quantum chemical calculations and did the main work on materials synthesis, device fabrication and both material and device characterization. Q.W. and H.Z. helped with the synthesis of the materials, while Y.X. and Y.Z. helped measure the device performances. X.C. helped revise the paper. L.D., D.Z. and T.H. discussed the results and wrote and revised the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Eli Zysman-Colman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–33, Tables 1–7 and Note 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, T., Wang, Q., Zhang, H. et al. Delocalizing electron distribution in thermally activated delayed fluorophors for high-efficiency and long-lifetime blue electroluminescence. Nat. Mater. 23, 1523–1530 (2024). https://doi.org/10.1038/s41563-024-02004-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-024-02004-w
This article is cited by
-
High-efficiency and long-lifetime deep-blue phosphorescent OLEDs using deuterated exciplex-forming host
Nature Communications (2025)
-
Exceptionally high brightness and long lifetime of efficient blue OLEDs for programmable active-matrix display
Light: Science & Applications (2025)