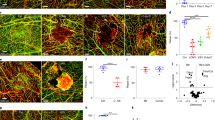
Abstract
Loss of endothelial integrity and vascular leakage are central features of sepsis pathogenesis; however, no effective therapeutic mechanisms for preserving endothelial integrity are available. Here we show that, compared to dermal microvessels, brain microvessels resist infection by Neisseria meningitidis, a bacterial pathogen that causes sepsis and meningitis. By comparing the transcriptional responses to infection in dermal and brain endothelial cells, we identified angiopoietin-like 4 as a key factor produced by the brain endothelium that preserves blood–brain barrier integrity during bacterial sepsis. Conversely, angiopoietin-like 4 is produced at lower levels in the peripheral endothelium. Treatment with recombinant angiopoietin-like 4 reduced vascular leakage, organ failure and death in mouse models of lethal sepsis and N. meningitidis infection. Protection was conferred by a previously uncharacterized ___domain of angiopoietin-like 4, through binding to the heparan proteoglycan, syndecan-4. These findings reveal a potential strategy to prevent endothelial dysfunction and improve outcomes in patients with sepsis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text, extended data and Supplementary Information. The RNA-seq raw files are accessible in the European Nucleotide Archive under the accession number PRJEB58351. Correspondence and requests for materials should be addressed to S.B. Source data are provided with this paper.
Code availability
No custom code was developed or used in the analysis of data presented in this manuscript.
References
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Cavaillon, J. M., Singer, M. & Skirecki, T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 12, e10128 (2020).
Jarczak, D., Kluge, S. & Nierhaus, A. Sepsis-pathophysiology and therapeutic concepts. Front. Med. 8, 628302 (2021).
Vincent, J. L., Ince, C. & Pickkers, P. Endothelial dysfunction: a therapeutic target in bacterial sepsis? Expert Opin. Ther. Targets 25, 733–748 (2021).
Klein, R. S. & Hunter, C. A. Protective and pathological immunity during central nervous system infections. Immunity 46, 891–909 (2017).
Bernard, S. C. et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 20, 725–731 (2014).
Maissa, N. et al. Strength of Neisseria meningitidis binding to endothelial cells requires highly-ordered CD147/beta2-adrenoceptor clusters assembled by alpha-actinin-4. Nat. Commun. 8, 15764 (2017).
Le Guennec, L. et al. Receptor recognition by meningococcal type IV pili relies on a specific complex N-glycan. Proc. Natl Acad. Sci. USA 117, 2606–2612 (2020).
Le Guennec, L., Coureuil, M., Nassif, X. & Bourdoulous, S. Strategies used by bacterial pathogens to cross the blood–brain barrier. Cell. Microbiol. 22, e13132 (2020).
Dos Santos Souza, I., Ziveri, J., Bouzinba-Segard, H., Morand, P. & Bourdoulous, S. Meningococcus, this famous unknown. C. R. Biol. 344, 127–143 (2021).
Pron, B. et al. Interaction of Neisseria maningitidis with the components of the blood–brain barrier correlates with an increased expression of PilC. J. Infect. Dis. 176, 1285–1292 (1997).
Mairey, E. et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood–brain barrier. J. Exp. Med. 203, 1939–1950 (2006).
Brandtzaeg, P. & van Deuren, M. Classification and pathogenesis of meningococcal infections. Methods Mol. Biol. 799, 21–35 (2012).
Giannotta, M., Trani, M. & Dejana, E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013).
Boardman, R. et al. Activation of Notch signaling by soluble Dll4 decreases vascular permeability via a cAMP/PKA-dependent pathway. Am. J. Physiol. Heart Circ. Physiol. 316, H1065–H1075 (2019).
London, N. R. et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci. Transl. Med. 2, 23ra19 (2010).
Hoppstadter, J. & Ammit, A. J. Role of dual-specificity phosphatase 1 in glucocorticoid-driven anti-inflammatory responses. Front. Immunol. 10, 1446 (2019).
Zhu, P., Goh, Y. Y., Chin, H. F., Kersten, S. & Tan, N. S. Angiopoietin-like 4: a decade of research. Biosci. Rep. 32, 211–219 (2012).
Yang, X., Cheng, Y. & Su, G. A review of the multifunctionality of angiopoietin-like 4 in eye disease. Biosci. Rep. 38, BSR20180557 (2018).
Fernandez-Hernando, C. & Suarez, Y. ANGPTL4: a multifunctional protein involved in metabolism and vascular homeostasis. Curr. Opin. Hematol. 27, 206–213 (2020).
Weksler, B., Romero, I. A. & Couraud, P. O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10, 16 (2013).
Weksler, B. B. et al. Blood–brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874 (2005).
Carbone, C. et al. Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int. J. Mol. Sci. 19, 431 (2018).
Chomel, C. et al. Interaction of the coiled-coil ___domain with glycosaminoglycans protects angiopoietin-like 4 from proteolysis and regulates its antiangiogenic activity. FASEB J. 23, 940–949 (2009).
Yau, M. H. et al. A highly conserved motif within the NH2-terminal coiled-coil ___domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J. Biol. Chem. 284, 11942–11952 (2009).
Ge, H. et al. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J. Biol. Chem. 279, 2038–2045 (2004).
Goligorsky, M. S. & Sun, D. Glycocalyx in endotoxemia and sepsis. Am. J. Pathol. 190, 791–798 (2020).
Kirsch, N. et al. Angiopoietin-like 4 Is a Wnt signaling antagonist that promotes LRP6 turnover. Dev. Cell 43, 71–82 e6 (2017).
Join-Lambert, O. et al. Meningococcal interaction to microvasculature triggers the tissular lesions of purpura fulminans. J. Infect. Dis. 208, 1590–1597 (2013).
Denis, K. et al. Targeting type IV pili as an antivirulence strategy against invasive meningococcal disease. Nat. Microbiol. 4, 972–984 (2019).
Flemming, S. et al. Soluble VE-cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis. Cardiovasc. Res. 107, 32–44 (2015).
Dolmatova, E. V., Wang, K., Mandavilli, R. & Griendling, K. K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 117, 60–73 (2021).
Chong, H. C. et al. Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol. Ther. 22, 1593–1604 (2014).
Le Jan, S. et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am. J. Pathol. 162, 1521–1528 (2003).
Huang, R. L. et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 118, 3990–4002 (2011).
Li, L. et al. Angiopoietin-like 4 increases pulmonary tissue leakiness and damage during influenza pneumonia. Cell Rep. 10, 654–663 (2015).
Zhang, B., Xu, X., Chu, X., Yu, X. & Zhao, Y. Protective effects of angiopoietin-like 4 on the blood–brain barrier in acute ischemic stroke treated with thrombolysis in mice. Neurosci. Lett. 645, 113–120 (2017).
Galaup, A. et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl Acad. Sci. USA 103, 18721–18726 (2006).
Bouleti, C. et al. Protective effects of angiopoietin-like 4 on cerebrovascular and functional damages in ischaemic stroke. Eur. Heart J. 34, 3657–3668 (2013).
Coureuil, M. et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science 325, 83–87 (2009).
Martins Gomes, S. F. et al. Induced pluripotent stem cell-derived brain endothelial cells as a cellular model to study Neisseria meningitidis infection. Front. Microbiol. 10, 1181 (2019).
Soares, M. P., Teixeira, L. & Moita, L. F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 17, 83–96 (2017).
McCarville, J. L. & Ayres, J. S. Disease tolerance: concept and mechanisms. Curr. Opin. Immunol. 50, 88–93 (2018).
Ishiguro, K. et al. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J. Biol. Chem. 276, 47483–47488 (2001).
Cazes, A. et al. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ. Res. 99, 1207–1215 (2006).
Hubers, C. et al. Primary tumor-derived systemic nANGPTL4 inhibits metastasis. J. Exp. Med. 220, e20202595 (2023).
Wang, L. et al. Therapeutic peptides: current applications and future directions. Signal Transduct. Target. Ther. 7, 48 (2022).
Nassif, X. et al. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol. Microbiol. 8, 719–725 (1993).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Kopylova, E., Noe, L. & Touzet, H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28, 3211–3217 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
R Core Team. R: A Language and Environment for Statistical Computing https://www.R-project.org/ (R Foundation for Statistical Computing, 2018).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015).
Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2019).
Acknowledgements
We thank L. Slimani and K. Henri from the core facility Imageries du Vivant Université Paris Cité for their expert technical help in micro-computed tomography, M. Favier of the histology facility, T. Guilbert of the imaging facility and K. Bailly and M. Andrieu of the cytometry facility of the Institut Cochin for their expert technical help. We thank Pr J. Pallud (Sainte-Anne Hospital, Paris) for providing the human brain tissues, F. Glacial and V. Vauthier (Brainplotting) for harvesting the primary HBMEC, and we thank M. Ingersoll (Institut Cochin) for careful review of the manuscript. The following funders provided support for this study: Agence Nationale de la Recherche ANR-14-IFEC14-0006 (S.B. and X.N.), Fondation pour la Recherche Médicale Equipe Grant EQU202003010400 (S.B.), Université Paris Cité IDEX UP 2020-S-I-001 (S.B.), Inserm transfert MAT-PI-19477-A-02 (S.B.), Austrian Science Fond FWF grant I 2191 (T.R.), Fondation pour la Recherche Médicale post-doctoral (J.Z.) and doctoral (L.L.G) fellowships and Université Paris Cité doctoral fellowship (I.d.S.S.). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.Z., L.L.G. and S.B. Methodology: J.Z., L.L.G., I.d.S.S., J.-P.B., S.M.W., Y.D., Y.S., E.L.S., H.B.-S., C.F., I.C., N.P., B.I., F.L. and T.S. Investigation: J.Z., L.L.G. and I.d.S.S. Scientific discussion: S.B., X.N., J.Z., L.L.G., P.C.M., C.F., M.C. and T.R. Funding acquisition: S.B., X.N. and T.R. Project administration: S.B. Supervision: S.B. Writing—original draft: S.B. and J.Z. Writing—review and editing: S.B., X.N., I.d.S.S., J.Z. and C.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Christian Schwerk, Sebastian Weis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Brain microvascular endothelial cells have intrinsic capacities to regulate inflammation in response to meningococcal infection.
a, A schematic representation of the protocols used in vitro to conduct the RNA sequencing analysis on primary human dermal (HDMEC) or brain (HBMEC) microvascular endothelial cells, created with BioRender.com. Cells were either uninfected or infected with N. meningitidis 2C4.3 for 3 h, mRNA were extracted and a global transcriptional analysis was performed to identify the differentially expressed genes (DEGs, see method, n = 3 samples per condition). The number of upregulated (red) and downregulated genes (blue) are indicated. Bottom, Venn diagram representing the DEG repartition in both cell types. b, Percentage of meningococcal read abundances in the sequenced samples showing similar levels of infection in both cell types. Error bars show mean ± s.e.m. c, Log2Fold changes of the 101 genes commonly regulated in both HDMEC and HBMEC upon infection (see details in Supplementary Table 3). d, Radar plots showing the production of inflammatory factors in supernatants of uninfected and infected HDMEC or HBMEC. Supernatants were collected after 3 h of infection and factors were measured with multiplex electrochemiluminescent immunoassays. Data are presented as relative fold changes from one representative experiment performed in quadruplicate with at least two independent biological replicates using two different donors.
Extended Data Fig. 2 ANGPTL4 produced by brain microvascular endothelial cells preserves endothelial integrity in response to meningococcal infection.
a, hCMEC/D3 were either uninfected or infected with N. meningitidis 2C4.3, cell supernatants were collected after 3 h of infection and the production of ANGPTL4 was quantified with electrochemiluminescent immunoassay. Error bars show mean ± s.e.m from one experiment performed in triplicate. b, hCMEC/D3 were either uninfected or infected with N. meningitidis 2C4.3 for 3- to 9-h. Cells were fixed and stained for cellular VE-cadherin, bacteria, and DAPI and analysed by fluorescent microscopy. The images are representative of VE-cadherin staining of two independent experiments performed in triplicate (scale bars, 50 μm). c, Extended data of the images shown in Fig. 1f (scale bars, 50 μm).
Extended Data Fig. 3 Interaction of the N-terminal part of ANGPTL4 with Syndecan-4 protects dermal microvascular endothelial cells from meningococcal infection.
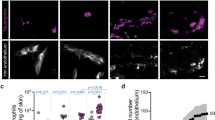
a-d, HDMEC either uninfected or infected with N. meningitidis 2C4.3 for 30 min were treated with 1 μg mL−1 of rhANGPTL4 or vehicle for 2h30. a, Cells were fixed and stained for cellular actin, VE-cadherin and DAPI and analysed by fluorescent microscopy (scale bars, 50 μm). b, c, Quantifications of VE-cadherin enrichment at intercellular junctions (b) and of intercellular spaces area (c) were performed with ImageJ software (n = 15 fields from n = 2 independent experiments). Error bars show mean ± s.e.m.; Ordinary one-way ANOVA Tukey’s multiple comparison test ****P < 0.0001; NS, not significant (NS1 = 0.994, NS2 = 0.9999). d, Infected HDMEC treated with 1 μg.mL−1 of rhANGPTL4 or vehicle for 2 h and 30 min were lysed and the number of adherent bacteria was quantified (CFU.ml−1). Error bars show mean ± s.d. from n = 2 independent experiments performed in duplicate; Unpaired t-test (two-tailed) NS, not significant (NS = 0.9357). e,f, Uninfected or infected HDMEC were treated with 1 μg.mL−1 of rhANGPTL4, rhANGPTL3, rhANGPTL8 or vehicle for 2 h and 30 min. Quantifications of VE-cadherin enrichment at intercellular junctions (d) and of intercellular spaces area (e) were performed with ImageJ software;. Error bars show mean ± s.e.m (n = 14 fields from n = 2 independent experiments). Ordinary one-way ANOVA Tukey’s multiple comparison test ****P < 0.001; NS, not significant (NS1 = 0.9993, NS2 > 0.9999, NS3 = 0.5346, NS4 = 0.9221). g, Extended data of the images shown in Fig. 2a (scale bars, 50 μm). h, mRNA levels of SDC4 in uninfected and infected HDMEC or HBMEC are represented as normalized values in transcripts per million (TPM). Error bars show mean ± s.e.m. from one experiment performed in triplicate.
Extended Data Fig. 4 Syndecan-4 expression is required to promote vascular protection by ANGPTL4.
a-e, HDMEC were transfected with small interfering RNA (siRNA) against SDC4 or non-targeting control siRNA (CTL). 48 h post-transfection, cells were either uninfected or infected with N. meningitidis 2C4.3 for 30 min and treated with 1 μg.mL−1 of rhANGPTL4 or vehicle for 2 h and 30 min. a, SDC4 depletion efficiency was evaluated by immunoblotting using β-tubulin as a loading control. Bottom: shown are the ratio between SCD4 and β-tubulin signals. b, c, Cells were fixed and stained for VE-cadherin and DAPI and analysed by fluorescent microscopy (scale bars, 50 μm). d,e, Quantifications of VE-cadherin enrichment at intercellular junctions and of intercellular spaces area were performed with ImageJ software. Error bars show mean ± s.e.m. (n = 10 fields from n = 2 independent experiments); Unpaired t-test Welch’s correction (two-tailed) ****P < 0.001; NS, not significant (NS1 = 0.7432, NS2 = 3703, NS3 = 0.6109, NS4 = 0.538).
Extended Data Fig. 5 Antibody neutralisation or siRNA depletion of SDC4 in hCMEC/D3 cells induces the loss of cell integrity in response to meningococcal infection.
a-e, hCMECs/D3 cells were either uninfected or infected with N. meningitidis 2C4.3 for 30 min and treated with 10 μg.mL−1 of anti-ANGPTL4 or anti-SDC4 antibodies for 2 h and 30 min. a, Cells were fixed and stained for VE-cadherin, Actin and DAPI and analysed by fluorescent microscopy. Representative images of 3 independent experiments are shown (scale bars, 50 μm). b, c, Quantifications of VE-cadherin enrichment at intercellular junctions and of intercellular spaces area were performed with ImageJ software. Error bars show mean ± s.e.m. (n = 15 fields from n = 2 independent experiments); Ordinary one-way ANOVA Tukey’s multiple comparison test ****P < 0.001; NS, not significant (NS1 = 0.9978, NS2 = 0.6648). d, Apoptosis assay. Error bars show mean ± s.e.m. (n = 2 independent experiments performed in triplicate). Ordinary one-way ANOVA Tukey’s multiple comparison test performed at 9 h compared to the uninfected conditions: *P < 0.05, NS, not significant (P > 0.05; NS1 = 0.9372, NS2 = 0.9941); or compared to the infected condition: § P < 0.05, NS, not significant (P > 0.05; NS3 = 0.9911). e-i, hCMEC/D3 cells were transfected with small interfering RNA (siRNA) against ANGPTL4, SDC4, or SDC1 or with a non-targeting control siRNA. 48 h post-transfection, cells were either uninfected or infected with N. meningitidis 2C4.3 for 30 min and treated with 1 μg.mL−1 of rhANGPTL4 or vehicle for 2 h and 30 min. e, ANGPLT4 depletion efficiency was evaluated by measuring the release of soluble ANGPTL4 in the cell supernatants. Error bars show mean ± s.e.m. from one experiment performed in duplicate. f, SDC4 and SDC1 depletion efficiency was evaluated by immunoblotting using clathrin as a loading control. Bottom: shown are the ratio between SCD4 and clathrin signals. g, Cells were fixed and stained for VE-cadherin, bacteria and DAPI and analysed by fluorescent microscopy. The images are representative of VE-cadherin staining in two independent experiments performed in duplicate (scale bars, 50 μm). h, Quantifications of VE-cadherin enrichment at intercellular junctions were performed with ImageJ software. Error bars show mean ± s.e.m; (n = 9 to 24 fields from n = 2 experiments performed in duplicate. Ordinary one-way ANOVA Tukey’s multiple comparison test ****P < 0.0001; *P < 0.05; NS, not significant (NS1 = 0.9508, NS2 = 0.4929, NS3 > 0.9999). i, Production of inflammatory markers in the cell supernatants. Error bars show mean ± s.e.m. of one representative experiment performed in triplicate. Ordinary one-way ANOVA Tukey’s multiple comparison test; ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 NS, not significant (NS1 = 0.4623, NS2 = 0.3255).
Extended Data Fig. 6 Antibody neutralisation of ANGPTL4 or SDC4 in primary human brain microvascular endothelial cells promotes vascular alteration and inflammation in response to meningococcal infection.
a-c, HBMEC grown on cell filters were either uninfected or infected with N. meningitidis 2C4.3 for 30 min and treated with 10 μg.mL−1 of anti-ANGPTL4 or anti-SDC4 antibodies or vehicle for 2h30. a, Transendothelial electrical resistance (TEER) was measured. Shown are the result of one representative experiment of two independent experiments performed in triplicate. Error bars show mean ± s.e.m; Ordinary one-way ANOVA Tukey’s multiple comparison test **P < 0.001; *P < 0.05. b, Cells were fixed and stained for VE-cadherin, Bacteria and DAPI and analysed by fluorescent microscopy. Representative images of 3 independent experiments are shown. c, Production of human cytokines (TNF, IL-6 and IL-8) and markers of vascular inflammation (E-selectin, ICAM-1, VCAM-1) or vascular alteration (Endoglin, Thrombomodulin) in the cell supernatants was quantified with multiplex electrochemiluminescent immunoassays. Shown are the result of one representative experiment performed in triplicate. Error bars show mean ± s.e.m; One-way ANOVA Dunnett’s multiple comparison test ****P < 0.0001; **P < 0.01; *P < 0.05; NS, not significant (NS1 = 0.9449, NS2 = 0.5652, NS3 = 0.3368, NS4 = 0.3843, NS5 = 0.6827, NS6 = 0.2803).
Extended Data Fig. 7 Vascular protection is conferred by a conserved functional ___domain in the N-terminal part of ANGPTL4.
a, Structure prediction of Peptide 4 was provided using PSIPRED Workbench (http://bioinf.cs.ucl.ac.uk/psipred/). b, Sequence homology between human and mouse ANGPTL4; a.a. 66-80 encompassing the functional motif are highlighted in yellow. c, HDMEC either uninfected or infected with N. meningitidis 2C4.3 for 30 min were treated with 1 μg.mL−1 of recombinant human or mouse ANGPTL4 or vehicle for 2 h and 30 min. Cells were fixed and stained for cellular VE-cadherin, Bacteria and DAPI and analysed by fluorescent microscopy (scale bars, 50 μm). Quantification of VE-cadherin enrichment at intercellular junctions were performed with ImageJ software (n = 5 to 6 fields from one representative experiment performed in duplicate). Error bars show mean ± s.e.m.; One-way ANOVA Tukey’s multiple comparison test ****P < 0.0001; NS, not significant (NS1 = 0.3515, NS2 = 0.8094).
Extended Data Fig. 8 The protective effects on thrombosis, vascular injury and inflammation are specific to ANGPTL4 and rely on the binding motif (aa 66-80) in its N-terminus ___domain.
a-f, SCID mice grafted with human skins were infected intravenously with N. meningitidis 2C4.3 (5 × 106 bacteria) or received vehicle. A period of 30 min after bacterial challenge, (b-d) mice received 1 µg of recombinant human ANGPTL3 or ANGPTL8 intravenously or the vehicle as a control (n = 3 mice per group); e,f, mice received 1 µg of nANGPTL4, Peptides P1, P9 or P10 intravenously or the vehicle as a control (n = 3 mice per group and this experiment was performed twice with n = 3 mice per group, for nANGPTL4 and P1 using skin from two different donors). Mice were killed at 4 h post-challenge. a, A schematic representation of the protocols used in vivo. b,e, Bacterial colonization and c,f, Thrombus formation within the skin grafts were assessed by immunofluorescence analysis and quantified as described in Fig. 3. Error bars show mean ± s.e.m.; 10 to 60 vessels per mouse were analysed; One-way ANOVA Tukey’s multiple comparison test, *** P < 0.001; NS, not significant (NS1 > 0.9999, NS2 = 0.264, NS3 = 0.9151, NS4 = 0.6748, NS5 = 0.7817). d, Production of human cytokines (IL-6 and IL-8) and markers of vascular inflammation (E-selectin, ICAM-1, VCAM-1) in the mouse plasma was quantified with multiplex electrochemiluminescent immunoassays: one point corresponds to one mouse. Error bars show the mean ± s.e.m. from one representative experiment; Ordinary one-way ANOVA Dunnett’s multiple comparison test. *P < 0.05; **P < 0.01; NS, not significant (NS1 = 0.3605, NS2 = 0.9183, NS3 = 0.089, NS4 = 0.6388, NS5 = 0.9434, NS6 = 0.9999, NS7 = 0.4262, NS8 = 0.9828, NS9 = 0.1948, NS10 = 0.6607, NS11 = 0.3228, NS12 = 0.2243).
Extended Data Fig. 9 The protective effects of ANGPTL4 against LPS-induced endotoxemia rely on the binding motif (aa 66-80) in its N-terminus ___domain.
a-e, Balb/c mice received LPS (5 mg.kg−1, i.p.) or vehicle as a control; 30 min after LPS challenge, mice received rhANGPTL4, nANGPTL4, peptide P1, or peptide P9 (2 µg, intravenously), or vehicle as a control. a, d, e, Shown are the body temperature and body weight monitored over 72 h. b, Production of mouse TNF and IL-6 and c, production of soluble mouse VE-cadherin in the mouse plasma were quantified with multiplex electrochemiluminescent immunoassays: one point corresponds to one mouse. Error bars show the mean ± s.d. from one (b) or two (c) representative experiment; Ordinary one-way ANOVA Tukey’s multiple comparison test, ****P < 0.0001; ***P < 0.001; **P < 0.01.
Extended Data Fig. 10 The protective effects of ANGPTL4 against LPS-induced endotoxemia rely on the binding motif (aa 66-80) in its N-terminus ___domain.
a, b, Balb/c mice received LPS (5 mg kg−1, i.p.) or vehicle as a control; 30 min after LPS challenge, mice received cANGPTL4, peptide P10 (2 µg, intravenously), or vehicle as a control. Left: Survival curves (n = 6 to 12 mice per group): NS, not significant (P > 0.05), two-sided log-rank Mantel−Cox survival analysis. Middle and right: body temperature and body weight monitored over 72 h.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Table
Supplementary Table 1: List of the genes regulated in HDMECs upon infection (comparison between infected HDMEC and uninfected HDMEC). Supplementary Table 2: List of the genes regulated in HBMECs upon infection (comparison between infected HBMEC and uninfected HBMEC). Supplementary Table 3: List of the 101 genes commonly regulated in both HDMEC and HBMEC upon infection (common between Supplementary Tables 3 and 4). Supplementary Table 4: List of the 40 genes specifically regulated in HBMEC upon infection. Supplementary Table 5: Comparison between uninfected HBMEC and uninfected HDMEC. Supplementary Table 6: Antibodies used in this study.
Source data
Source Data Fig. 1
Source data for Fig. 1b,d,e,g–i.
Source Data Fig. 2
Source data for Fig. 2b–f.
Source Data Fig. 3
Source data for Fig. 3b,c.
Source Data Fig. 4
Source data for Fig. 4b,d–h.
Source Data Fig. 5
Source data for Fig. 5b–d,f,i,l.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1b,d.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2a.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3b–f,h.
Source Data Extended Data Fig. 4
Unprocessed western blots for Fig. 4a and source data for Extended Data Fig. 4d,e.
Source Data Extended Data Fig. 5
Unprocessed western blots for Fig. 5g and source data for Extended Data Fig. 5b–e,g–i.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6a,c.
Source Data Extended Data Fig. 7
Source data for Extended Data Fig. 7c.
Source Data Extended Data Fig. 8
Source data for Extended Data Fig. 8b–f.
Source Data Extended Data Fig. 9
Source data for Extended Data Fig. 9a–e.
Source Data Extended Data Fig. 10
Source data for Extended Data Fig. 10a,b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ziveri, J., Le Guennec, L., dos Santos Souza, I. et al. Angiopoietin-like 4 protects against endothelial dysfunction during bacterial sepsis. Nat Microbiol 9, 2434–2447 (2024). https://doi.org/10.1038/s41564-024-01760-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-024-01760-4