Abstract
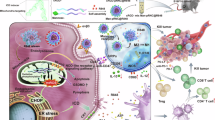
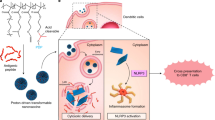
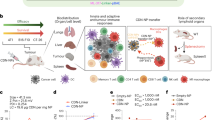
Cancer metastases and recurrence after surgical resection remain an important cause of treatment failure. Here we demonstrate a general strategy to fabricate personalized nanovaccines based on a cationic fluoropolymer for post-surgical cancer immunotherapy. Nanoparticles formed by mixing the fluoropolymer with a model antigen ovalbumin, induce dendritic cell maturation via the Toll-like receptor 4 (TLR4)-mediated signalling pathway, and promote antigen transportation into the cytosol of dendritic cells, which leads to an effective antigen cross-presentation. Such a nanovaccine inhibits established ovalbumin-expressing B16-OVA melanoma. More importantly, a mix of the fluoropolymer with cell membranes from resected autologous primary tumours synergizes with checkpoint blockade therapy to inhibit post-surgical tumour recurrence and metastases in two subcutaneous tumour models and an orthotopic breast cancer tumour. Furthermore, in the orthotopic tumour model, we observed a strong immune memory against tumour rechallenge. Our work offers a simple and general strategy for the preparation of personalized cancer vaccines to prevent post-operative cancer recurrence and metastasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors upon reasonable request.
References
Klevorn, L. E. & Teague, R. M. Adapting cancer immunotherapy models for the real world. Trends Immunol. 37, 354–363 (2016).
Littman, D. R. Releasing the brakes on cancer immunotherapy. Cell 162, 1186–1190 (2015).
Wang, C., Ye, Y., Hu, Q., Bellotti, A. & Gu, Z. Tailoring biomaterials for cancer immunotherapy: emerging trends and future outlook. Adv. Mater. 29, 1606036 (2017).
Lu, J. et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 8, 1811 (2017).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Chao, Y. et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2, 611–621 (2018).
Weichselbaum, R. R., Liang, H., Deng, L. & Fu, Y. X. Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol. 14, 365–379 (2017).
Chen, Q. et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 14, 89–97 (2019).
Wang, C. et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 1, 0011 (2017).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Yarchoan, M., Johnson, B. A. III, Lutz, E. R., Laheru, D. A. & Jaffee, E. M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 17, 209–222 (2017).
Guo, Y., Lei, K. & Tang, L. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front. Immunol. 9, 1499 (2018).
Zhu, G., Zhang, F., Ni, Q., Niu, G. & Chen, X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 11, 2387–2392 (2017).
Luo, M. et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 12, 648–654 (2017).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2016).
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1, 362–368 (1999).
Cruz, F. M., Colbert, J. D., Merino, E., Kriegsman, B. A. & Rock, K. L. The biology and underlying mechanisms of cross-presentation of exogenous antigens on MHC-I molecules. Annu. Rev. Immunol. 35, 149–176 (2017).
Lv, S. et al. Nanoparticles encapsulating hepatitis B virus cytosine-phosphate-guanosine induce therapeutic immunity against HBV infection. Hepatology 59, 385–394 (2014).
Yang, J., Zhang, Q., Chang, H. & Cheng, Y. Surface-engineered dendrimers in gene delivery. Chem. Rev. 115, 5274–5300 (2015).
Zhang, Z. et al. The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat. Commun. 9, 1377 (2018).
Cametti, M., Crousse, B., Metrangolo, P., Milani, R. & Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 41, 31–42 (2012).
Stewart, M. P., Langer, R. & Jensen, K. F. Intracellular delivery by membrane disruption: mechanisms, strategies, and concepts. Chem. Rev. 118, 7409–7531 (2018).
Janeway, C. A. Jr & Bottomly, K. Signals and signs for lymphocyte responses. Cell 76, 275–285 (1994).
Folks, T. M. et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl Acad. Sci. USA 86, 2365–2368 (1989).
Dienz, O. & Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 130, 27–33 (2009).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
He, W. et al. Re-polarizing myeloid-derived suppressor cells (MDSCs) with cationic polymers for cancer immunotherapy. Sci. Rep. 6, 24506 (2016).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Yamamoto, A. et al. Bafilomycin A prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23, 33–42 (1998).
Wang, M., Liu, H., Li, L. & Cheng, Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat. Commun. 5, 3053 (2014).
Bevan, M. J. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 4, 595–602 (2004).
Yang, R. et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano 12, 5121–5129 (2018).
Kroll, A. V. et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv. Mater. 29, 1703969 (2017).
Ochyl, L. J. et al. PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials 182, 157–166 (2018).
Grosso, J. F. & Jure-Kunkel, M. N. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immunol. Res. 13, 5 (2013).
D’Alise, A. M. et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat. Commun. 10, 2688 (2019).
Li, A. W. et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 17, 528–534 (2018).
Xu, L. et al. Morphologically virus-like fullerenol nanoparticles act as the dual-functional nanoadjuvant for HIV-1 vaccine. Adv. Mater. 25, 5928–5936 (2013).
Issadore, D. et al. Self-assembled magnetic filter for highly efficient immunomagnetic separation. Lab Chip 11, 147–151 (2011).
Xu, J. et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano 11, 4463–4474 (2017).
Acknowledgements
This work was partially supported by the National Key Research and Development (R&D) Program of China (2016YFA0201200, 2017YFE0131700 and 2019YFA0904500), the National Natural Science Foundation of China (51525203, 21725402 and 51761145041), the 111 Project, the Jiangsu Social Development Project (BE2019658), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (19KJA310008) and the Collaborative Innovation Center of Suzhou Nano Science and Technology (Nano-CIC). The authors thank H. Liu for B16-OVA tumour cells and X. Wang for the OT-I mice. Our gratitude also goes to Y. Huang for valuable comments and suggestions.
Author information
Authors and Affiliations
Contributions
Z.L., Y.C., R.P. and J.X. conceived the project and designed the experiments. Z.L. and R.P. supervised the project. Z.L., R.P. and J.X. wrote the manuscript. J.X., J.L., Q.Z., Z.Y., Z.C., P.P., C.W., H.W., Z.D. and Y.C. carried out the experiments. J.L. assisted in taking and analysing the microscope images. C.W. and K.Y. supported the operation and analysis of the flow cytometer. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Jeffrey Hubbell, Helder A. Santos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27 and Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Xu, J., Lv, J., Zhuang, Q. et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 15, 1043–1052 (2020). https://doi.org/10.1038/s41565-020-00781-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-00781-4
This article is cited by
-
An immune activator encapsulating PD-L1 siRNA for augmented immune checkpoint blockade immunotherapy through Zn2+ overload triggered pyroptosis
Journal of Nanobiotechnology (2025)
-
In situ construction of heterojunctions to regulate the biodegradation behavior of copper carriers for tumor-specific cuproptosis-enhanced sono-immunotherapy
Journal of Nanobiotechnology (2025)
-
Trigger inducible tertiary lymphoid structure formation using covalent organic frameworks for cancer immunotherapy
Nature Communications (2025)
-
Autophagosomes coated in situ with nanodots act as personalized cancer vaccines
Nature Nanotechnology (2025)
-
Neoantigen enriched biomimetic nanovaccine for personalized cancer immunotherapy
Nature Communications (2025)