Abstract
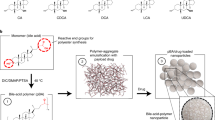
Contrary to current insulin formulations, endogenous insulin has direct access to the portal vein, regulating glucose metabolism in the liver with minimal hypoglycaemia. Here we report the synthesis of an amphiphilic diblock copolymer comprising a glucose-responsive positively charged segment and polycarboxybetaine. The mixing of this polymer with insulin facilitates the formation of worm-like micelles, achieving highly efficient absorption by the gastrointestinal tract and the creation of a glucose-responsive reservoir in the liver. Under hyperglycaemic conditions, the polymer triggers a rapid release of insulin, establishing a portal-to-peripheral insulin gradient—similarly to endogenous insulin—for the safe regulation of blood glucose. This insulin formulation exhibits a dose-dependent blood-glucose-regulating effect in a streptozotocin-induced mouse model of type 1 diabetes and controls the blood glucose at normoglycaemia for one day in non-obese diabetic mice. In addition, the formulation demonstrates a blood-glucose-lowering effect for one day in a pig model of type 1 diabetes without observable hypoglycaemia, showing promise for the safe and effective management of type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the results of this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Katsarou, A. et al. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 3, 17016 (2017).
Mathieu, C., Martens, P. J. & Vangoitsenhoven, R. One hundred years of insulin therapy. Nat. Rev. Endocrinol. 17, 715–725 (2021).
Mitragotri, S., Burke, P. A. & Langer, R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 13, 655–672 (2014).
Geho, W. B. The importance of the liver in insulin replacement therapy in insulin-deficient diabetes. Diabetes 63, 1445–1447 (2014).
Brown, T. D., Whitehead, K. A. & Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 5, 127–148 (2020).
Lee, J. S. et al. Metabolic and immunomodulatory control of type 1 diabetes via orally delivered bile-acid-polymer nanocarriers of insulin or rapamycin. Nat. Biomed. Eng. 5, 983–997 (2021).
Veiseh, O., Tang, B. C., Whitehead, K. A., Anderson, D. G. & Langer, R. Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug Discov. 14, 45–57 (2015).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2, 16075 (2016).
Bakh, N. A. et al. Glucose-responsive insulin by molecular and physical design. Nat. Chem. 9, 937–944 (2017).
Wang, J. et al. Glucose-responsive insulin and delivery systems: innovation and translation. Adv. Mater. 32, 1902004 (2020).
Mo, R., Jiang, T., Di, J., Tai, W. & Gu, Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 43, 3595–3629 (2014).
Wang, Z., Wang, J., Kahkoska, A. R., Buse, J. B. & Gu, Z. Developing insulin delivery devices with glucose responsiveness. Trends Pharmacol. Sci. 42, 31–44 (2021).
Gu, Z. et al. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano 7, 6758–6766 (2013).
Chen, Z. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 14, 86–93 (2018).
Podual, K., Doyle, F. J. & Peppas, N. A. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J. Control. Release 67, 9–17 (2000).
Kitano, S., Koyama, Y., Kataoka, K., Okano, T. & Sakurai, Y. A novel drug delivery system utilizing a glucose responsive polymer complex between poly (vinyl alcohol) and poly (N-vinyl-2-pyrrolidone) with a phenylboronic acid moiety. J. Control. Release 19, 161–170 (1992).
Hisamitsu, I., Kataoka, K., Okano, T. & Sakurai, Y. Glucose-responsive gel from phenylborate polymer and poly (vinyl alcohol): prompt response at physiological pH through the interaction of borate with amino group in the gel. Pharm. Res. 14, 289–293 (1997).
Matsumoto, A. et al. A synthetic approach toward a self-regulated insulin delivery system. Angew. Chem. Int. Ed. 51, 2124–2128 (2012).
Chou, D. H.-C. et al. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl Acad. Sci. USA 112, 2401–2406 (2015).
Wang, J. et al. Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Sci. Adv. 5, eaaw4357 (2019).
Yu, J. et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 4, 499–506 (2020).
Wang, J. et al. Injectable biodegradable polymeric complex for glucose-responsive insulin delivery. ACS Nano 15, 4294–4304 (2021).
Matsumoto, A. et al. Synthetic ‘smart gel’ provides glucose-responsive insulin delivery in diabetic mice. Sci. Adv. 3, eaaq0723 (2017).
Zhang, J. et al. Week-long normoglycaemia in diabetic mice and minipigs via a subcutaneous dose of a glucose-responsive insulin complex. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-023-01138-7 (2023).
Brownlee, M. & Cerami, A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science 206, 1190–1191 (1979).
Wang, J. et al. Glucose transporter inhibitor-conjugated insulin mitigates hypoglycemia. Proc. Natl Acad. Sci. USA 116, 10744–10748 (2019).
Yao, Y., Ji, K., Wang, Y., Gu, Z. & Wang, J. Materials and carriers development for glucose-responsive insulin. Acc. Mater. Res. 3, 960–970 (2022).
Chu, J. N. & Traverso, G. Foundations of gastrointestinal-based drug delivery and future developments. Nat. Rev. Gastroenterol. Hepatol. 19, 219–238 (2022).
Yang, T. et al. Ligand-switchable nanoparticles resembling viral surface for sequential drug delivery and improved oral insulin therapy. Nat. Commun. 13, 6649 (2022).
Xi, Z. et al. Dual-modified nanoparticles overcome sequential absorption barriers for oral insulin delivery. J. Control. Release 342, 1–13 (2022).
Drucker, D. J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 19, 277–289 (2020).
Yang, Y. et al. Recent advances in oral and transdermal protein delivery systems. Angew. Chem. Int. Ed. 62, e202214795 (2023).
Baryakova, T. H., Pogostin, B. H., Langer, R. & McHugh, K. J. Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems. Nat. Rev. Drug Discov. 22, 387–409 (2023).
Lamson, N. G., Berger, A., Fein, K. C. & Whitehead, K. A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. 4, 84–96 (2020).
Lagarrigue, P., Moncalvo, F. & Cellesi, F. Non-spherical polymeric nanocarriers for therapeutics: the effect of shape on biological systems and drug delivery properties. Pharmaceutics 15, 32 (2023).
Ji, K. et al. Material design for oral insulin delivery. Med X 1, 7 (2023).
Yu, J. et al. Glucose-responsive oral insulin delivery for postprandial glycemic regulation. Nano Res. 12, 1539–1545 (2019).
Wang, A. et al. Liver-target and glucose-responsive polymersomes toward mimicking endogenous insulin secretion with improved hepatic glucose utilization. Adv. Funct. Mater. 30, 1910168 (2020).
Xiao, Y. et al. Glucose-responsive oral insulin delivery platform for one treatment a day in diabetes. Matter 4, 3269–3285 (2021).
Han, X. et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat. Nanotechnol. 15, 605–614 (2020).
Lu, Y. et al. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2, 318–325 (2018).
Banerjee, A., Qi, J., Gogoi, R., Wong, J. & Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 238, 176–185 (2016).
Xiao, W. et al. Highly sensitive colorimetric detection of a variety of analytes via the Tyndall effect. Anal. Chem. 91, 15114–15122 (2019).
Cone, R. A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 61, 75–85 (2009).
Pridgen, E. M. et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci. Transl. Med. 5, 213ra167 (2013).
Acosta-Rodríguez, V. et al. Circadian alignment of early onset caloric restriction promotes longevity in male c57BL/6J mice. Science 376, 1192–1202 (2022).
Yang, R. et al. A glucose-responsive insulin therapy protects animals against hypoglycemia. JCI Insight 3, e97476 (2018).
Qiu, Y. et al. Long-lasting designer insulin with glucose-dependent solubility markedly reduces risk of hypoglycemia. Adv. Ther. (Weinh.) 2, 1900128 (2019).
Ayala, J. E. et al. Hyperinsulinemic–euglycemic clamps in conscious, unrestrained mice. J. Vis. Exp. 16, e3188 (2011).
Cao, Z., Zhang, L. & Jiang, S. Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir 28, 11625–11632 (2012).
Fan, W. et al. Mucus penetrating and cell-binding polyzwitterionic micelles as potent oral nanomedicine for cancer drug delivery. Adv. Mater. 34, 2109189 (2022).
Zou, J.-J. et al. Efficient oral insulin delivery enabled by transferrin-coated acid-resistant metal–organic framework nanoparticles. Sci. Adv. 8, eabm4677 (2022).
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2022YFE0202200, J.W.), JDRF (2-SRA-2021-1064-M-B, Z.G.; 2-SRA-2022-1159-M-B, J.W.), the Key Project of Science and Technology Commission of Zhejiang Province (2024C03083, Z.G.; 2024C03085, J.W.), Zhejiang University’s start-up packages and the Starry Night Science Fund at Shanghai institute for Advanced Study of Zhejiang University (SN-ZJU-SIAS-009, J.W.). A.R.K. is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (KL2TR002490, J.W.). The project was supported by the Clinical and Translational Science Award program of the National Center for Advancing Translational Science, National Institutes of Health (UL1TR002489, J.W.). We appreciate the help from J. Pan and D. Wu of the Research and Service Center (College of Pharmaceutical Science, Zhejiang University) for technical support, G. Z. and Y. Zhang (Cryo-EM centre, Zhejiang University) for processing the samples for electron microscopy and D. Xu, M. Zhang, S. Xiong and D. Chen (Disease Simulation and Animal Model Platform of Liangzhu Laboratory) for taking care of the minipigs.
Author information
Authors and Affiliations
Contributions
Z.G., J.W., Y.S. and J.B.B. conceived and designed the study. K.J., Xiangqian Wei, J.Z., J.X., Xinwei Wei, Y.Z., W.L., Y.W., Y.Y., S.M. and Y.L. conducted experiments and obtained related data. X.H., S.W., Z.Z., J.Y., G.X. and Z.L. gave experimental operation and theoretical guidance of mice experiments. K.J., Xiangqian Wei, J.Z. and J.X. conducted minipigs experiments and provided theoretical support. Z.G., J.W., Y.S., K.J., J.Z., Xiangqian Wei, A.R.K., J.B.B. and J.X. analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
Z.G. is the co-founder of Zenomics Inc., Zcapsule Inc. and μZen Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Kåre Birkeland and Nicholas Hunt for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 BG-regulating effects in diabetic minipigs.
BG of diabetic minipigs treated with the insulin capsules (oral), the PPF-ins capsules (oral) or Lantus (s.c.). The insulin dose of oral formulations was set to 4.2 U/kg. The Lantus dose was set to 0.3 U/kg.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24.
Supplementary Data 1
Supplementary statistical source data.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data Extended Data Fig. 1
Statistical source data for Extended Data Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, K., Wei, X., Kahkoska, A.R. et al. An orally administered glucose-responsive polymeric complex for high-efficiency and safe delivery of insulin in mice and pigs. Nat. Nanotechnol. 19, 1880–1891 (2024). https://doi.org/10.1038/s41565-024-01764-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-024-01764-5