Abstract
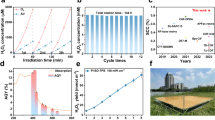
Selective asymmetric oxidation of glycerol (GLY) to hydroxypyruvic acid (HPA) offers an attractive approach for chiral drug synthesis, but this process is highly challenging. Here we develop a photocatalytic method to achieve heterogeneous selective photooxidation of GLY to HPA over rubidium (Rb) and iridium (Ir) catalytic pairs decorated on a poly(heptazine imide) framework. The Rb sites effectively adsorb GLY molecules through the terminal –OH groups, thus inhibiting their oxidation during photoreaction, while the Ir sites enhance the oxygen reduction reaction and the in situ generated surficial oxygen-reduction radicals on Ir can protect the reactive C-centred radical intermediates produced during photooxidation. The spatial arrangement of Rb and Ir sites facilitates hydrogen extraction—an essential rate-determining step for GLY photooxidation—and protects C3 radical intermediates from overoxidation. This photocatalytic system achieves a remarkable productivity for HPA synthesis (~8,000 μmol of HPA per gram of photocatalyst per hour) under visible-light illumination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All source data for figures in the Article, extended data and Supplementary Information will be uploaded to the Nature publication system. The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
OECD–FAO Agricultural Outlook 2020–2029 (OECD, 2020).
Werpy, T. A., Holladay, J. E. & White, J. F. Top Value Added Chemicals From Biomass: Results of Screening for Potential Candidates from Sugars and Synthesis Gas (US Department of Energy, 2004).
Dodekatos, G., Schünemann, S. & Tüysüz, H. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal. 8, 6301–6333 (2018).
Pagliaro, M., Ciriminna, R., Kimura, H., Rossi, M., & Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 46, 4434–4440 (2007).
Dias da Silva Ruy, A. et al. Market prospecting and assessment of the economic potential of glycerol from biodiesel. In Biotechnological Applications of Biomass (eds Basso, T. P. et al.) Ch. 11 (IntechOpen, 2020).
Katryniok, B. et al. Selective catalytic oxidation of glycerol: perspectives for high value chemicals. Green Chem. 13, 1960–1979 (2011).
Sheng, H. et al. Linear paired electrochemical valorization of glycerol enabled by the electro-Fenton process using a stable NiSe2 cathode. Nat. Catal. 5, 716–725 (2022).
Kobori, Y., Myles, D. C. & Whitesides, G. M. Substrate specificity and carbohydrate synthesis using transketolase. J. Org. Chem. 57, 5899–5907 (1992).
Liu, Z., Xiao, C., Lin, S., Tittmann, K. & Dai, S. Multifaceted role of the substrate phosphate group in transketolase catalysis. ACS Catal. 14, 355–365 (2024).
Horecker, B. L., Hurwitz, J. & Smyrniotis, P. Z. Xylulose 5-phosphate and the formation of sedoheptulose 7-phosphate with liver transketolase. J. Am. Chem. Soc. 78, 692–694 (1956).
Munos, J. W., Pu, X., Mansoorabadi, S. O., Kim, H. J. & Liu, H.-W. A secondary kinetic isotope effect study of the 1-deoxy-d-xylulose-5-phosphate reductoisomerase-catalyzed reaction: evidence for a retroaldol–aldol rearrangement. J. Am. Chem. Soc. 131, 2048–2049 (2009).
Shaeri, J., Wohlgemuth, R. & Woodley, J. M. Semiquantitative process screening for the biocatalytic synthesis of d-xylulose-5-phosphate. Org. Process Res. Dev. 10, 605–610 (2006).
Cai, G. et al. Thermodynamic investigation of inhibitor binding to 1-deoxy-d-xylulose-5-phosphate reductoisomerase. ACS Med. Chem. Lett. 3, 496–500 (2012).
Kumar, M., Meena, B., Yu, A., Sun, C. & Challapalli, S. Advancements in catalysts for glycerol oxidation via photo-/electrocatalysis: a comprehensive review of recent developments. Green Chem. 25, 8411–8443 (2023).
Xiao, Y. et al. Selective photoelectrochemical oxidation of glycerol to glyceric acid on (002) facets exposed WO3 nanosheets. Angew. Chem. Int. Ed. 63, e202319685 (2024).
Liu, D. et al. Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat. Commun. 10, 1779 (2019).
Teng, Z. et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 4, 374–384 (2021).
Teng, Z. et al. Atomically dispersed low-valent Au boosts photocatalytic hydroxyl radical production. Nat. Chem. 16, 1250–1260 (2024).
Savateev, A., Pronkin, S., Willinger, M. G., Antonietti, M. & Dontsova, D. Towards organic zeolites and inclusion catalysts: Heptazine imide salts can exchange metal cations in the solid state. Chem. Asian J. 12, 1517–1522 (2017).
Wirnhier, E. et al. Poly(triazine imide) with intercalation of lithium and chloride ions [(C3N3)2(NHxLi1−x)3⋅LiCl]: a crystalline 2D carbon nitride network. Chem. Eur. J. 17, 3213–3221 (2011).
Schlomberg, H. et al. Structural Insights into poly(heptazine imides): a light-storing carbon nitride material for dark photocatalysis. Chem. Mater. 31, 7478–7486 (2019).
Lee, J. H. et al. Carbon dioxide mediated, reversible chemical hydrogen storage using a Pd nanocatalyst supported on mesoporous graphitic carbon nitride. J. Mater. Chem. A 2, 9490–9495 (2014).
Zhang, J.-R. et al. Accurate K-edge X-ray photoelectron and absorption spectra of g-C3N4 nanosheets by first-principles simulations and reinterpretations. Phys. Chem. Chem. Phys. 21, 22819–22830 (2019).
Wang, W. et al. Potassium-Ion-assisted regeneration of active cyano groups in carbon nitride nanoribbons: visible-light-driven photocatalytic nitrogen reduction. Angew. Chem. Int. Ed. 58, 16644–16650 (2019).
Kessler, F. K. et al. Functional carbon nitride materials—design strategies for electrochemical devices. Nat. Rev. Mater. 2, 17030 (2017).
Lin, L., Yu, Z. & Wang, X. Crystalline carbon nitride semiconductors for photocatalytic water splitting. Angew. Chem. Int. Ed. 58, 6164–6175 (2019).
Lin, L. et al. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 3, 649–655 (2020).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Bredas, J.-L. Mind the gap! Mater. Horiz. 1, 17–19 (2014).
Vogt, C. & Weckhuysen, B. M. The concept of active site in heterogeneous catalysis. Nat. Rev. Chem. 6, 89–111 (2022).
Wang, C., Wang, Z., Mao, S., Chen, Z. & Wang, Y. Coordination environment of active sites and their effect on catalytic performance of heterogeneous catalysts. Chin. J. Catal. 43, 928–955 (2022).
Wang, H., Cui, Y., Shi, J., Tao, X. & Zhu, G. Porous carbon supported Lewis acid–base sites as metal-free catalysts for the carbonylation of glycerol with urea. Appl. Catal. B 330, 122457 (2023).
An, Z. et al. Pt1 enhanced C–H activation synergistic with Ptn catalysis for glycerol cascade oxidation to glyceric acid. Nat. Commun. 13, 5467 (2022).
Luo, L. et al. Selective photoelectrocatalytic glycerol oxidation to dihydroxyacetone via enhanced middle hydroxyl adsorption over a Bi2O3-incorporated catalyst. J. Am. Chem. Soc. 144, 7720–7730 (2022).
Mörsdorf, J.-M. & Ballmann, J. Coordination-induced radical generation: selective hydrogen atom abstraction via controlled Ti–C σ-bond homolysis. J. Am. Chem. Soc. 145, 23452–23460 (2023).
Bellotti, P., Huang, H. M., Faber, T. & Glorius, F. Photocatalytic late-stage C–H functionalization. Chem. Rev. 123, 4237–4352 (2023).
Zhang, X. et al. Fast modulation of d-band holes quantity in the early reaction stages for boosting acidic oxygen evolution. Angew. Chem. Int. Ed. 62, e202308082 (2023).
Hao, Y. et al. Electrode/electrolyte synergy for concerted promotion of electron and proton transfers toward efficient neutral water oxidation. Angew. Chem. Int. Ed. 62, e202303200 (2023).
Dai, X. et al. Aerobic oxidative synthesis of formamides from amines and bioderived formyl surrogates. Angew. Chem. Int. Ed. 63, e202402241 (2024).
Zhang, L., Ma, L., Yuan, J., Zhang, X.-M. & Tang, Z. Tuning band structures of Hf-PCN-224(M) for β-carbonyl C(sp3)-H bond activation and difunctionalization: tandem C(sp3) radical cross-coupling through photoredox. Appl. Catal. B 321, 122049 (2023).
Teng, Z. et al. Atomically isolated Sb(CN)3 on sp2-c-COFs with balanced hydrophilic and oleophilic sites for photocatalytic C–H activation. Sci. Adv. 10, eadl5432 (2024).
Chang, C. R., Yang, X. F., Long, B. & Li, J. A water-promoted mechanism of alcohol oxidation on a Au(111) surface: understanding the catalytic behavior of bulk gold. ACS Catal. 3, 1693–1699 (2013).
Huang, X., Guo, Y., Zou, Y. & Jiang, J. Electrochemical oxidation of glycerol to hydroxypyruvic acid on cobalt(oxy) hydroxide by high-valent cobalt redox centers. Appl. Catal. B 309, 121247 (2022).
Kim, H. J., Lee, J., Green, S. K., Huber, G. W. & Kim, W. B. Selective glycerol oxidation by electrocatalytic dehydrogenation. ChemSusChem 7, 1051–1056 (2014).
Jedsukontorn, T., Ueno, T., Saito, N. & Hunsom, M. Narrowing band gap energy of defective black TiO2 fabricated by solution plasma process and its photocatalytic activity on glycerol transformation. J. Alloys Compd. 757, 188–199 (2018).
Choi, Y.-B., Nunotani, N., Morita, K. & Imanaka, N. Production of hydroxypyruvic acid by glycerol oxidation over Pt/CeO2-ZrO2-Bi2O3-PbO/SBA-16 catalysts. Catalysts 12, 69 (2022).
Jedsukontorn, T., Saito, N. & Hunsom, M. Photocatalytic behavior of metal-decorated TiO2 and their catalytic activity for transformation of glycerol to value added compounds. Mol. Catal. 432, 160–171 (2017).
Sun, Y. et al. PtBi intermetallic compounds with enhanced stability towards base-free selective oxidation of glycerol. Ind. Eng. Chem. Res. 62, 17503–17512 (2023).
Dou, J. et al. Carbon supported Pt9Sn1 nanoparticles as an efficient nanocatalyst for glycerol oxidation. Appl. Catal. B 180, 78–85 (2016).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Wu, Q. et al. A metal-free photocatalyst for highly efficient hydrogen peroxide photoproduction in real seawater. Nat. Commun. 12, 483 (2021).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Lu, Y. et al. Solar-driven highly selective conversion of glycerol to dihydroxyacetone using surface atom engineered BiVO4 photoanodes. Nat. Commun. 15, 5475 (2024).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
We thank the financial support from the City University of Hong Kong startup fund (grant no. 9020003), ITF–RTH – Global STEM Professorship (grant no. 9446006), JC STEM lab of Advanced CO2 Upcycling (grant no. 9228005), National Natural Science Foundation of China (grant nos. 11904235, 22125604, 22372102 and 22436003), the National Key Research and Development Program of China (grant no. 2021YFA1600800), Educational Commission of Guangdong Province (grant no. 839-0000013131), Shenzhen Science and Technology Program (grant no. RCJC20200714114434086), Guangdong Basic and Applied Basic Research Foundation (grant no. 2024A1515010976), Shenzhen Peacock Plan (grant no. 20210802524B) and Research Team Cultivation Program of Shenzhen University (grant no. 2023QNT013). We also thank C. Chen (King Abdullah University of Science and Technology) and Y. Han (King Abdullah University of Science and Technology).
Author information
Authors and Affiliations
Contributions
Z.T. and B.L. conceptualized the project. D.Z., J.L., C.S. and B.L. supervised the project. Z.T. and Z.Z. synthesized the catalysts, conducted the catalytic tests and the related data processing, and performed materials characterization and analysis with help from Y.T., L.Y., L.H., C.W., Q.Z., O.T., H.Y., J.X. and J.D. Z.T. and Z.Z. performed the theoretical study. N.J. performed the microscopy measurement. Z.T. and B.L. wrote the manuscript with support from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Zaizhu Lou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 X-ray diffraction patterns of the as-prepared Rb-PHI and Ir0.5Rb-PHI.
a, Structural model of Rb-PHI along the zone axis of [001]. b, Structural model of Rb-PHI along the zone axis of [010]. c, XRD pattern of the as-prepared Rb-PHI. d, XRD pattern of the as-prepared Ir0.5Rb-PHI.
Extended Data Fig. 2 Influence of Ir and Rb species on chemical states of C and N.
a-b, Normalized carbon (a) and nitrogen (b) K-edge XANES spectra for PHI, Rb-PHI and Ir0.5Rb-PHI.
Extended Data Fig. 3 Extended X-ray absorption fine structure (EXAFS) spectra of Rb-PHI, Ir0.5-PHI and Ir0.5Rb-PHI.
a, Fourier transform extended X-ray absorption fine structure (FT-EXAFS) spectra of Rb-OH, Rb-PHI, Ir0.5Rb-PHI and RbCl. b, k3-weighted k-space spectrum of Rb for Ir0.5Rb-PHI. c, Fourier transform extended X-ray absorption fine structure (FT-EXAFS) spectra of IrO2, Ir0.5-PHI, Ir0.5Rb-PHI and Ir foil. d, k3-weighted k-space spectrum of Ir for Ir0.5Rb-PHI.
Extended Data Fig. 4 Surface chemical states of C, N, Rb and Ir in Rb-PHI, Ir0.5-PHI and Ir0.5Rb-PHI.
a-c, High-resolution XPS spectra of N 1 s (a), C 1 s (b) and Rb 3 d (c) for Rb-PHI and Ir0.5Rb-PHI. d, High-resolution XPS spectra of Ir 4 f for Ir0.5-PHI and Ir0.5Rb-PHI. The almost no shift in the Rb 3 d XPS spectrum is attributed to the notably large content of Rb (~7% wt.%) species in Ir0.5Rb-PHI, which cannot be remarkably affected by the Ir species with a low concentration (~0.5 wt.%). On the other hand, the shift of the Ir 4 f XPS spectrum is notable for Ir0.5Rb-PHI as compared to that for Ir0.5-PHI.
Extended Data Fig. 5 Post-structural characterization of Ir0.5Rb-PHI.
a, The Ir and Rb contents before and after the photoreaction. b, TEM image of Ir0.5Rb-PHI after 30 h of photoreaction. c, High-resolution image of b. d, HAADF-STEM image of Ir0.5Rb-PHI after the 30 h of photoreaction. e, HAADF-STEM image of Ir0.5Rb-PHI before photoreaction and the corresponding EDX mappings. f, HAADF-STEM image of Ir0.5Rb-PHI after 30 h of photoreaction and the corresponding EDX mappings. g-h, High-resolution Rb 3 d (g) and Ir 4 f (h) XPS spectra for Ir0.5Rb-PHI after 30 h of photoreaction.
Extended Data Fig. 6 Density of states.
a-b, Total density of states (TDOS), partial density of states (PDOS) and overlapped density of states (ODOS) for a, Rb incorporated hexagonal heptazine imide (Rb-HHI) and b, Ir and Rb co-incorporated hexagonal heptazine imide (IrRb-HHI).
Extended Data Fig. 7 Adsorption of HPA, GLD and GLA on Rb-PHI and IrRb-PHI.
a, Adsorption energy of GLY-end-on (GLY-T), GLA, GLY-side-on (GLY-S) and GLD on Rb-PHI. b, Adsorption energy of GLY-T, GLA, GLY-S and GLD on IrRb-PHI. GLY-T refers to the adsorption configuration of attaching the terminal-OH of GLY to Rb site on Rb-PHI and IrRb-PHI. GLY-S refers to the adsorption configuration of attaching the secondary-OH of GLY to Rb site on Rb-PHI and IrRb-PHI. GLA refers to the adsorption configuration of attaching the terminal-OH of GLA to Rb site on Rb-PHI and IrRb-PHI. HPA refers to the adsorption configuration of attaching the terminal -OH groups of HPA to Rb site on Rb-PHI and IrRb-PHI.
Extended Data Fig. 8 Exploration of oxygen reduction intermediates.
Raman spectra recorded over Rb-PHI in O2 saturated GLY aqueous solution (0.1 M) during photoreaction for 0, 5, 10, 15 and 20 min from bottom to top.
Extended Data Fig. 9 Photooxidation of GLY for selective HPA synthesis under various O2 pressure.
a, Production rate under the reaction condition of 0.2 M GLY, 298 K, 2 atm O2 (left) and 1 atm O2 (right). c, Production rate under the reaction condition of 0.5 M GLY, 298 K, 5 atm O2 (left) and 1 atm O2 (right). e, Production rate under the reaction condition of 0.1 M GLY, 298 K, 5 atm O2 (left) and 1 atm O2 (right). b, d, f, HPA selectivity and GLY conversion under the reaction condition of 0.2 M GLY at 298 K in 2 atm O2 (left) and 1 atm O2 (right) (b), 0.5 M GLY at 298 K in 5 atm O2 (left) and 1 atm O2 (right) (d) and 0.1 M GLY at 298 K in 5 atm O2 (left) and 1 atm O2 (right) (f).
Extended Data Fig. 10 Partial density of states for various oxidation intermediates.
a-b, PDOS of CH2OH-·COH-COOH on Rb-PHI (a) and IrRb-PHI (b). c-d, PDOS of CH2OH-CHO on Rb-PHI (c) and IrRb-PHI (d).
Supplementary information
Supplementary Information
Supplementary Figs. 1–64, Tables 1–14 and Notes 1–12.
Supplementary Data 1
Source data of supplementary figures.
Source data
Source Data Fig. 1
Source data of Fig. 1.
Source Data Fig. 2
Source data of Fig. 2.
Source Data Fig. 3
Source data of Fig. 3.
Source Data Fig. 4
Source data of Fig. 4.
Source Data Fig. 5
Source data of Fig. 5.
Source Data Extended Data Fig. 1
Source data of Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data of Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data of Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data of Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Source data of Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Source data of Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source data of Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Source data of Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Source data of Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Source data of Extended Data Fig. 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teng, Z., Zhang, Z., Tu, Y. et al. Asymmetric photooxidation of glycerol to hydroxypyruvic acid over Rb–Ir catalytic pairs on poly(heptazine imides). Nat. Nanotechnol. 20, 815–824 (2025). https://doi.org/10.1038/s41565-025-01897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-025-01897-1