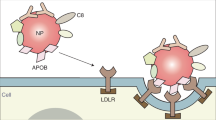
Abstract
Liver macrophages capture circulating nanoparticles and reduce their delivery to target organs. Serum proteins adsorb to the nanoparticle surface after administration. However, the adsorbed serum proteins and their cognate cell receptors for removing nanoparticles from the bloodstream have not been linked. Here we use a multi-omics strategy to identify the adsorbed serum proteins binding to specific liver macrophage receptors. We discovered six absorbed serum proteins that bind to two liver macrophage receptors. Nanoparticle physicochemical properties can affect the degree of the six serum proteins adsorbing to the surface, the probability of binding to cell receptors and whether the liver removes the nanoparticle from circulation. Identifying the six adsorbed proteins allowed us to engineer decoy nanoparticles that prime the liver to take up fewer therapeutic nanoparticles, enabling more nanoparticles for targeting extrahepatic tissues. Elucidating the molecular interactions governing the nanoparticle journey in vivo will enable us to control nanoparticle delivery to diseased tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main paper and the Supplementary Information contain all data supporting the findings of this study. The raw data are available from the corresponding author upon reasonable request. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD061236. Genomics sequencing data have been deposited to the NCBI SRA genomics databases with the accession number PRJNA1228635.
References
Zhang, Y.-N., Poon, W., Tavares, A. J., McGilvray, I. D. & Chan, W. C. W. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. J. Control. Release 240, 332–348 (2016).
Ngo, W. et al. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv. Drug Deliv. Rev. 185, 114238 (2022).
Wang, G. et al. High-relaxivity superparamagnetic iron oxide nanoworms with decreased immune recognition and long-circulating properties. ACS Nano 8, 12437–12449 (2014).
Nagayama, S. et al. Fetuin mediates hepatic uptake of negatively charged nanoparticles via scavenger receptor. Int. J. Pharm. 329, 192–198 (2007).
Binnemars-Postma, K. A., Ten Hoopen, H. W., Storm, G. & Prakash, J. Differential uptake of nanoparticles by human M1 and M2 polarized macrophages: protein corona as a critical determinant. Nanomedicine 11, 2889–2902 (2016).
Montizaan, D. et al. Genome-wide forward genetic screening to identify receptors and proteins mediating nanoparticle uptake and intracellular processing. Nat. Nanotechnol. 19, 1022–1031 (2024).
Lin, Z. P., Ngo, W., Mladjenovic, S. M., Wu, J. L. Y. & Chan, W. C. W. Nanoparticles bind to endothelial cells in injured blood vessels via a transient protein corona. Nano Lett. 23, 1003–1009 (2023).
Ngo, W. et al. Identifying cell receptors for the nanoparticle protein corona using genome screens. Nat. Chem. Biol. https://doi.org/10.1038/s41589-022-01093-5 (2022).
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A. & Chan, W. C. W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134, 2139–2147 (2012).
Walkey, C. D. et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8, 2439–2455 (2014).
Capriotti, A. L. et al. Differential analysis of ‘protein corona’ profile adsorbed onto different nonviral gene delivery systems. Anal. Biochem. 419, 180–189 (2011).
Toth, C. A. & Thomas, D. P. Liver endocytosis and Kupffer cells. Hepatology 16, 255–266 (1992).
Gref, R. et al. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B 18, 301–313 (2000).
Tavares, A. J. et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl Acad. Sci. USA 114, E10871–E10880 (2017).
von Mering, C. et al. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 31, 258–261 (2003).
Szklarczyk, D. et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Uhlén, M. et al. The human secretome. Sci. Signal. 12, eaaz0274 (2019).
Hart, T. et al. Evaluation and design of genome-wide CRISPR/SpCas9 knockout screens. G3 7, 2719–2727 (2017).
Liu, T. et al. Optimization of differentiation and transcriptomic profile of THP-1 cells into macrophage by PMA. PLoS ONE 18, e0286056 (2023).
MacParland, S. A. et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 4383 (2018).
Chanput, W., Mes, J. J. & Wichers, H. J. THP-1 cell line: an in vitro cell model for immune modulation approach. Int. Immunopharmacol. 23, 37–45 (2014).
Starr, T., Bauler, T. J., Malik-Kale, P. & Steele-Mortimer, O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS ONE 13, e0193601 (2018).
Auwerx, J., Staels, B., Van Vaeck, F. & Ceuppens, J. L. Changes in IgG Fc receptor expression induced by phorbol 12-myristate 13-acetate treatment of THP-1 monocytic leukemia cells. Leuk. Res. 16, 317–327 (1992).
Lundqvist, M. et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA 105, 14265–14270 (2008).
de Boer, C. G., Ray, J. P., Hacohen, N. & Regev, A. MAUDE: inferring expression changes in sorting-based CRISPR screens. Genome Biol. 21, 134 (2020).
Colic, M. et al. Identifying chemogenetic interactions from CRISPR screens with DrugZ. Genome Med. 11, 52 (2019).
Matsumoto, A. et al. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc. Natl Acad. Sci. USA 87, 9133–9137 (1990).
Platt, N. & Gordon, S. Scavenger receptors: diverse activities and promiscuous binding of polyanionic ligands. Chem. Biol. 5, R193–R203 (1998).
Hezareh, M., Hessell, A. J., Jensen, R. C., van de Winkel, J. G. & Parren, P. W. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J. Virol. 75, 12161–12168 (2001).
Grage-Griebenow, E. et al. Human MO subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology 202, 42–50 (2000).
Bulut, Y. et al. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J. Biol. Chem. 280, 20961–20967 (2005).
Estruch, M. et al. CD14 and TLR4 mediate cytokine release promoted by electronegative LDL in monocytes. Atherosclerosis 229, 356–362 (2013).
Mukhopadhyay, S. et al. SR-A/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell surface limits TLR4 response to pathogens. Blood 117, 1319–1328 (2011).
Onyishi, C. U. et al. Toll-like receptor 4 and macrophage scavenger receptor 1 crosstalk regulates phagocytosis of a fungal pathogen. Nat. Commun. 14, 4895 (2023).
Thomas, P. D. et al. PANTHER: making genome-scale phylogenetics accessible to all. Protein Sci. 31, 8–22 (2022).
Mi, H., Muruganujan, A. & Thomas, P. D. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 41, D377–D386 (2013).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Bowdish, D. M. E. et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 5, e1000474 (2009).
Brown, M. S., Basu, S. K., Falck, J. R., Ho, Y. K. & Goldstein, J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J. Supramol. Struct. 13, 67–81 (1980).
Stringer, B., Imrich, A. & Kobzik, L. Lung epithelial cell (A549) interaction with unopsonized environmental particulates: quantitation of particle-specific binding and IL-8 production. Exp. Lung Res. 22, 495–508 (1996).
Means, N., Elechalawar, C. K., Chen, W. R., Bhattacharya, R. & Mukherjee, P. Revealing macropinocytosis using nanoparticles. Mol. Asp. Med. 83, 100993 (2022).
Harush-Frenkel, O., Debotton, N., Benita, S. & Altschuler, Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 353, 26–32 (2007).
Wang, Z., Tiruppathi, C., Minshall, R. D. & Malik, A. B. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 3, 4110–4116 (2009).
Wolfram, J. et al. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci. Rep. 7, 13738 (2017).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Manzanares, D. & Ceña, V. Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 12, 371 (2020).
Sousa de Almeida, M. et al. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 50, 5397–5434 (2021).
Zhang, S., Gao, H. & Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 9, 8655–8671 (2015).
Vácha, R., Martinez-Veracoechea, F. J. & Frenkel, D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett. 11, 5391–5395 (2011).
Morita, S.-Y., Deharu, Y., Takata, E., Nakano, M. & Handa, T. Cytotoxicity of lipid-free apolipoprotein B. Biochim. Biophys. Acta 1778, 2594–2603 (2008).
Ricklin, D. Manipulating the mediator: modulation of the alternative complement pathway C3 convertase in health, disease and therapy. Immunobiology 217, 1057–1066 (2012).
Forneris, F. et al. Regulators of complement activity mediate inhibitory mechanisms through a common C3b-binding mode. EMBO J. 35, 1133–1149 (2016).
Tavano, R. et al. C1q-mediated complement activation and C3 opsonization trigger recognition of stealth poly(2-methyl-2-oxazoline)-coated silica nanoparticles by human phagocytes. ACS Nano 12, 5834–5847 (2018).
Inturi, S. et al. Modulatory role of surface coating of superparamagnetic iron oxide nanoworms in complement opsonization and leukocyte uptake. ACS Nano 9, 10758–10768 (2015).
Dobrovolskaia, M. A., Aggarwal, P., Hall, J. B. & McNeil, S. E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 5, 487–495 (2008).
Wu, J. et al. Structure of complement fragment C3b–factor H and implications for host protection by complement regulators. Nat. Immunol. 10, 728–733 (2009).
Schöttler, S. et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 11, 372–377 (2016).
Cheng, C. et al. Recognition of lipoproteins by scavenger receptor class A members. J. Biol. Chem. 297, 100948 (2021).
Petithory, T. et al. Size-dependent internalization efficiency of macrophages from adsorbed nanoparticle-based monolayers. Nanomaterials 11, 1963 (2021).
Perry, J. L. et al. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 12, 5304–5310 (2012).
Capriotti, A. L. et al. Do plasma proteins distinguish between liposomes of varying charge density? J. Proteom. 75, 1924–1932 (2012).
Caracciolo, G., Pozzi, D., Capriotti, A. L., Cavaliere, C. & Laganà, A. Effect of DOPE and cholesterol on the protein adsorption onto lipid nanoparticles. J. Nanopart. Res. 15, 1498 (2013).
Pozzi, D. et al. Effect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale 6, 2782–2792 (2014).
Caracciolo, G. et al. The liposome–protein corona in mice and humans and its implications for in vivo delivery. J. Mater. Chem. B 2, 7419–7428 (2014).
O’Connell, D. J. et al. Characterization of the bionano interface and mapping extrinsic interactions of the corona of nanomaterials. Nanoscale 7, 15268–15276 (2015).
Caracciolo, G. et al. Lipid composition: a ‘key factor’ for the rational manipulation of the liposome–protein corona by liposome design. RSC Adv. 5, 5967–5975 (2015).
Huang, H. et al. An evaluation of blood compatibility of silver nanoparticles. Sci. Rep. 6, 25518 (2016).
Pisani, C. et al. Experimental separation steps influence the protein content of corona around mesoporous silica nanoparticles. Nanoscale 9, 5769–5772 (2017).
Arcella, A. et al. Brain targeting by liposome-biomolecular corona boosts anticancer efficacy of temozolomide in glioblastoma cells. ACS Chem. Neurosci. 9, 3166–3174 (2018).
Lai, W. et al. A protein corona adsorbed to a bacterial magnetosome affects its cellular uptake. Int. J. Nanomed. 15, 1481–1498 (2020).
Nierenberg, D. et al. Macromolecules absorbed from influenza infection-based sera modulate the cellular uptake of polymeric nanoparticles. Biomimetics 7, 219 (2022).
Baimanov, D. et al. Stereoselective coronas regulate the fate of chiral gold nanoparticles in vivo. Nanoscale Horiz. 8, 859–869 (2023).
Ashkarran, A. A. et al. Measurements of heterogeneity in proteomics analysis of the nanoparticle protein corona across core facilities. Nat. Commun. 13, 6610 (2022).
Ouyang, B. et al. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 19, 1362–1371 (2020).
Aldridge, W. N. Serum esterases. II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem. J. 53, 117–124 (1953).
Ramirez Reyes, J. M. J., Cuesta, R. & Pause, A. Folliculin: a regulator of transcription through AMPK and mTOR signaling pathways. Front. Cell Dev. Biol. 9, 667311 (2021).
Acknowledgements
This work was supported by the Canadian Institute of Health Research (FDN159932 and MOP-1301431), the NanoMedicines Innovation Network (2019-T3-01) and the Canadian Research Chairs Program (950-223824). We thank NSERC (J.L.Y.W. and Z.S.), the Ontario Graduate Scholarship (B.B., M.G.G.M. and Z.S.), Lorne F. Lambier, Q.C. Scholarship (Z.S.), the Cecil Yip Award (B.B., J.L.Y.W., Z.S. and A.G.F.), the Jennifer Dorrington Award (J.L.Y.W.), the Vanier Award (S.A.), the MAX Scholarship Fund (S.A.), the Adel S. Sedra graduate award (S.A.), the MD/PhD programme (S.A.) and the Barbara and Frank Milligan family (J.L.Y.W. and Z.S.) for student fellowships and scholarships. B.S. acknowledges the Doctoral Completion Award and NSERC CREATE grant for funding support. We would like to thank S. Mladjenovic for his assistance in imaging cells. In addition, we would like to thank SPARC BioCentre for help with protein identification, the Nanomedicine Fabrication Centre for ICP-MS, the Centre for Phenogenomics for histology and the Temerty Faculty of Medicine and SickKids Flow Cytometry Facilities for flow cytometry and cell sorting.
Author information
Authors and Affiliations
Contributions
B.B., W.N., J.M. and W.C.W.C. conceptualized the project. W.N., J.L.Y.W. and A.G.F. did a genome-wide CRISPR screen. B.B., W.N. and W.C.W.C. analysed the data. B.B. completed the inhibition experiments. B.B. and Z.P.L. completed the histology experiments. S.A. collected electron micrographs. B.B. did the protein corona identification and STRING analysis. B.B. and M.G.G.M. did the competition experiments. B.B. completed the meta-analysis. Z.S. and B.S. synthesized the liposomes. B.B. labelled and coated the nanoparticles. B.B. evaluated the decoys in vitro. B.B., Z.P.L. and M.G.G.M. evaluated the decoys in vivo. B.B. and W.C.W.C. wrote the paper. All authors participated in editing and revising the paper.
Corresponding author
Ethics declarations
Competing interests
W.C.W.C. consults for Metis Therapeutics, Merck, Moderna, Foresight Ventures, Luna Nanotech and Cystic Fibrosis Foundation. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Tables 1 and 2.
Supplementary Table 3
Raw data for complex tables including mass spectrometry and meta-analysis data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bussin, B., MacDuff, M.G.G., Ngo, W. et al. Discovering nanoparticle corona ligands for liver macrophage capture. Nat. Nanotechnol. (2025). https://doi.org/10.1038/s41565-025-01903-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-025-01903-6