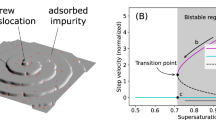
Abstract
Impurities critically influence crystallization, a process fundamental to both physical sciences and industrial engineering. However, understanding how impurity transport affects crystallization presents substantial experimental challenges. Here we visualized crystallization at the single-particle level for a relatively high concentration of impurities. We observed a bifurcation in growth modes—continuous growth or melting and recrystallization—governed by the ability of the system to remove impurity particles from the growth front. The initial nucleation configuration determines the crystal grain size and growth-front morphology, which in turn influence impurity transport. Small grains promote lateral impurity transport to grain boundaries, thus reducing impurity concentration and favouring continuous growth, whereas larger grains accumulate impurities, leading to melting and recrystallization. We reveal that the latter arises from the competition between crystallization and vitrification, which is a form of devitrification. This study provides insights into the relation between impurity concentration and crystallization pathways and highlights how the initial configuration shapes the final crystal morphology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. Source data are provided with this paper.
Code availability
The codes used in this study are available from the corresponding authors upon request.
References
Myerson, A. Handbook of Industrial Crystallization (Butterworth-Heinemann, 2002).
Chen, Q., Bae, S. C. & Granick, S. Directed self-assembly of a colloidal kagome lattice. Nature 469, 381–384 (2011).
Damasceno, P. F., Engel, M. & Glotzer, S. C. Predictive self-assembly of polyhedra into complex structures. Science 337, 453–457 (2012).
Gasser, U., Weeks, E. R., Schofield, A., Pusey, P. & Weitz, D. Real-space imaging of nucleation and growth in colloidal crystallization. Science 292, 258–262 (2001).
Pusey, P. N. & Van Megen, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 320, 340–342 (1986).
Zhu, J. et al. Crystallization of hard-sphere colloids in microgravity. Nature 387, 883–885 (1997).
Wang, H. et al. Freezing shrinkage dynamics and surface dendritic growth of floating refractory alloy droplets in outer space. Adv. Mater. 36, 2313162 (2024).
Wolde, P. R. T. & Frenkel, D. Enhancement of protein crystal nucleation by critical density fluctuations. Science 277, 1975–1978 (1997).
Tan, P., Xu, N. & Xu, L. Visualizing kinetic pathways of homogeneous nucleation in colloidal crystallization. Nat. Phys. 10, 73–79 (2014).
Kawasaki, T. & Tanaka, H. Formation of a crystal nucleus from liquid. Proc. Natl Acad. Sci. USA 107, 14036–14041 (2010).
Li, M. et al. Revealing thermally-activated nucleation pathways of diffusionless solid-to-solid transition. Nat. Commun. 12, 4042 (2021).
Wang, Z., Wang, F., Peng, Y., Zheng, Z. & Han, Y. Imaging the homogeneous nucleation during the melting of superheated colloidal crystals. Science 338, 87–90 (2012).
Peng, Y. et al. Two-step nucleation mechanism in solid–solid phase transitions. Nat. Mater. 14, 101–108 (2015).
Li, M., Chen, Y., Tanaka, H. & Tan, P. Revealing roles of competing local structural orderings in crystallization of polymorphic systems. Sci. Adv. 6, eaaw8938 (2020).
Arai, S. & Tanaka, H. Surface-assisted single-crystal formation of charged colloids. Nat. Phys. 13, 503–509 (2017).
Chen, Y. et al. Visualizing slow internal relaxations in a two-dimensional glassy system. Nat. Phys. 19, 969–977 (2023).
Meng, G., Paulose, J., Nelson, D. R. & Manoharan, V. N. Elastic instability of a crystal growing on a curved surface. Science 343, 634–637 (2014).
Chen, Y. et al. Morphology selection kinetics of crystallization in a sphere. Nat. Phys. 17, 121–127 (2021).
Irvine, W. T., Vitelli, V. & Chaikin, P. M. Pleats in crystals on curved surfaces. Nature 468, 947–951 (2010).
Hwang, H., Weitz, D. A. & Spaepen, F. Direct observation of crystallization and melting with colloids. Proc. Natl Acad. Sci. USA 116, 1180–1184 (2019).
Tegze, G. et al. Diffusion-controlled anisotropic growth of stable and metastable crystal polymorphs in the phase-field crystal model. Phys. Rev. Lett. 103, 035702 (2009).
Freitas, R. & Reed, E. J. Uncovering the effects of interface-induced ordering of liquid on crystal growth using machine learning. Nat. Commun. 11, 3260 (2020).
Mullins, W. W. & Sekerka, R. Stability of a planar interface during solidification of a dilute binary alloy. J. Appl. Phys. 35, 444–451 (1964).
Warren, J. A. & Langer, J. Prediction of dendritic spacings in a directional-solidification experiment. Phys. Rev. E 47, 2702 (1993).
Langer, J. S. Instabilities and pattern formation in crystal growth. Rev. Mod. Phys. 52, 1 (1980).
Losert, W., Shi, B. & Cummins, H. Evolution of dendritic patterns during alloy solidification: onset of the initial instability. Proc. Natl Acad. Sci. USA 95, 431–438 (1998).
Gránásy, L., Pusztai, T., Tegze, G., Warren, J. A. & Douglas, J. F. Growth and form of spherulites. Phys. Rev. E 72, 011605 (2005).
Watanabe, K., Kawasaki, T. & Tanaka, H. Structural origin of enhanced slow dynamics near a wall in glass-forming systems. Nat. Mater. 10, 512–520 (2011).
Zaccarelli, E. et al. Crystallization of hard-sphere glasses. Phys. Rev. Lett. 103, 135704 (2009).
Yanagishima, T., Russo, J. & Tanaka, H. Common mechanism of thermodynamic and mechanical origin for ageing and crystallization of glasses. Nat. Commun. 8, 15954 (2017).
Gao, Q. et al. Fast crystal growth at ultra-low temperatures. Nat. Mater. 20, 1431–1439 (2021).
Cacciuto, A., Auer, S. & Frenkel, D. Onset of heterogeneous crystal nucleation in colloidal suspensions. Nature 428, 404–406 (2004).
De Villeneuve, V. W. et al. Colloidal hard-sphere crystal growth frustrated by large spherical impurities. Science 309, 1231–1233 (2005).
Allahyarov, E., Sandomirski, K., Egelhaaf, S. U. & Löwen, H. Crystallization seeds favour crystallization only during initial growth. Nat. Commun. 6, 7110 (2015).
Farmanesh, S. et al. Specificity of growth inhibitors and their cooperative effects in calcium oxalate monohydrate crystallization. J. Am. Chem. Soc. 136, 367–376 (2014).
Schmidt, C. & Ulrich, J. Morphology prediction of crystals grown in the presence of impurities and solvents—an evaluation of the state of the art. J. Cryst. Growth 353, 168–173 (2012).
Kuvadia, Z. B. & Doherty, M. F. Effect of structurally similar additives on crystal habit of organic molecular crystals at low supersaturation. Cryst. Growth Des. 13, 1412–1428 (2013).
Zhao, M., Xiong, Y., Shang, Y. & Xu, X. Effect of nano-impurity on liquid/solid phase transition of water droplet. J. Mol. Liq. 398, 124232 (2024).
Shklovskii, B. I. & Efros, A. L. Electronic Properties of Doped Semiconductors, Vol. 45 (Springer, 2013).
Erwin, S. C. et al. Doping semiconductor nanocrystals. Nature 436, 91–94 (2005).
Zhao, J. et al. Structural and magnetic phase diagram of CeFeAsO1−xFx and its relation to high-temperature superconductivity. Nat. Mater. 7, 953–959 (2008).
Pramanik, S., Cherusseri, J., Baban, N. S., Sowntharya, L. & Kar, K. K. Metal Matrix Composites: Theory, Techniques, and Applications (Springer, 2017).
Zhang, S., Wang, Z., Guo, B. & Xu, J. Secondary nucleation in polymer crystallization: a kinetic view. Polym. Cryst. 4, e10173 (2021).
Cabrera, N. & Vermilyea, D. in Growth and Perfection of Crystals (eds Doremus, R. H. et al.) 393–410 (Wiley, 1958).
Granasy, L. et al. Growth of ‘dizzy dendrites’ in a random field of foreign particles. Nat. Mater. 2, 92–96 (2003).
Gránásy, L., Pusztai, T., Börzsönyi, T., Warren, J. A. & Douglas, J. F. A general mechanism of polycrystalline growth. Nat. Mater. 3, 645–650 (2004).
Warren, J. A., Pusztai, T., Környei, L. & Gránásy, L. Phase field approach to heterogeneous crystal nucleation in alloys. Phys. Rev. B 79, 014204 (2009).
Sangwal, K. Effects of impurities on crystal growth processes. Prog. Cryst. Growth Characteriz. Mater. 32, 3–43 (1996).
Kubota, N. Effect of impurities on the growth kinetics of crystals. Cryst. Res. Technol. 36, 749–769 (2001).
Stipp, A. & Palberg, T. Crystal growth kinetics in binary mixtures of model charged sphere colloids. Philos. Mag. 87, 899–908 (2007).
Steinhardt, P. J., Nelson, D. R. & Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B 28, 784 (1983).
Sanz, E. et al. Avalanches mediate crystallization in a hard-sphere glass. Proc. Natl Acad. Sci. USA 111, 75–80 (2014).
Yunker, P., Zhang, Z., Aptowicz, K. B. & Yodh, A. G. Irreversible rearrangements, correlated domains, and local structure in aging glasses. Phys. Rev. Lett. 103, 115701 (2009).
Ganapathi, D., Chakrabarti, D., Sood, A. & Ganapathy, R. Structure determines where crystallization occurs in a soft colloidal glass. Nat. Phys. 17, 114–120 (2021).
Cabane, B. et al. Hiding in plain view: colloidal self-assembly from polydisperse populations. Phys. Rev. Lett. 116, 208001 (2016).
Shinohara, M. et al. Recrystallization and zone melting of charged colloids by thermally induced crystallization. Langmuir 29, 9668–9676 (2013).
Schiøtz, J., Di Tolla, F. D. & Jacobsen, K. W. Softening of nanocrystalline metals at very small grain sizes. Nature 391, 561–563 (1998).
Dalal, M. A Textbook of Physical Chemistry, Vol. 1 (Dalal Institute, 2018).
Hsu, M. F., Dufresne, E. R. & Weitz, D. A. Charge stabilization in nonpolar solvents. Langmuir 21, 4881–4887 (2005).
Lechner, W. & Dellago, C. Accurate determination of crystal structures based on averaged local bond order parameters. J. Chem. Phys. 129, 114707 (2008).
Mickel, W., Kapfer, S. C., Schröder-Turk, G. E. & Mecke, K. Shortcomings of the bond orientational order parameters for the analysis of disordered particulate matter. J. Chem. Phys. 138, 044501, (2013).
Torquato, S. & Stillinger, F. H. Local density fluctuations, hyperuniformity, and order metrics. Phys. Rev. E 68, 041113 (2003).
Acknowledgements
P.T. acknowledges support from the National Natural Science Foundation of China (Grant Nos. 12425503, 12174071 and 12035004), the Space Application System of China Manned Space Program (Grant No. KJZ-YY-NLT0501), the Innovation Program of Shanghai Municipal Education Commission (Grant No. 2023ZKZD06) and the Shanghai Pilot Program for Basic Research-FuDan University (Grant No. 22TQ003). Q.G. acknowledges support from the China Postdoc Science Foundation (Grant No. BX20220072). H.T. acknowledges support from a Grant-in-Aid for Specially Promoted Research (Grant No. JP20H05619) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
H.T. and P.T. conceived and supervised the research and wrote the paper. Q.G., D.X. and Y.C. performed the experiments. Q.G., H.F., D.X., Y.C., H.T. and P.T. analysed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Dora Izzo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Spatial distribution of foreign particles and nuclei at initial configuration.
a,b, Initial distribution of nuclei and foreign particles for two growth modes. Foreign particles are labelled in pink. Left panels: Nuclei are shown in light blue. Right panels: Shaded areas represent the distribution of foreign particles within nuclei. c, Density variance 〈δρ2〉 of foreign particles at the initial stage of nucleation for both growth modes. Five samples of MR growth modes and four samples of CG mode are analyzed. The CG mode curve represents the mean values of four measurements, with error bars indicating their upper and lower bounds.
Extended Data Fig. 2 Defect types of foreign particles in BCC lattice.
a, Substitutional: A single foreign particle occupies a lattice site. b, Interstitial: A single foreign particle occupies an interstitial site. c, Substitutional interstitial pair: One foreign particle occupies a lattice site and the other an interstitial site. d,e, Defect chain: Substitutional-interstitial pairs form a defect chain, with crystal order decreasing as the number of foreign particles in the chain increases. f, Cluster: Foreign particles aggregate into a cluster, causing geometric frustration that impedes crystallization.
Extended Data Fig. 3 3D micro-structure of the melting region.
Evolution of the 3D structure in the melting region before, during, and after melting, with solid particles in olive green, liquid particles in blue, and foreign particles in pink.
Supplementary information
Supplementary Video 1
Morphological evolution of the growth front and the foreign-particle distribution in CG mode.
Supplementary Video 2
Morphological evolution of the growth front and the foreign-particle distribution in MR mode.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Q., Fang, H., Xiang, D. et al. Impact of impurities on crystal growth. Nat. Phys. 21, 938–946 (2025). https://doi.org/10.1038/s41567-025-02870-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-025-02870-4