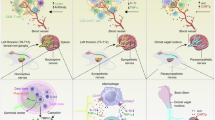
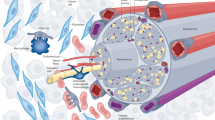
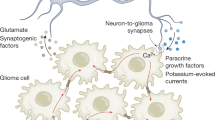
Abstract
The nervous and immune systems have co-evolved to detect and respond to internal and external threats, working together to restore homeostasis after tissue injury or infection. Sharing several receptors and ligands, they engage in direct cross-talk that substantially influences disease development. The emerging field of cancer neuro-immunity focuses on the intricate interactions between the nervous system, immune responses and tumour growth. Additional findings have revealed that nerve fibres infiltrating peripheral tumours can release neuromodulatory factors that shape both immune cell behaviour and tumour progression. Conversely, tumour-infiltrating immune cells can modify the activity of local neurons, including pain-transmitting nociceptive sensory neurons. Beyond sensory fibres, sympathetic signalling can foster immunosuppression by recruiting myeloid-derived suppressor cells and promoting T cell exhaustion. This Review summarizes current evidence on how neuronal signalling regulates peripheral antitumour immune responses within the tumour microenvironment. We describe the complex, reciprocal interactions among neurons, immune cells and malignant cells, highlighting the key parts played by the peripheral nervous system in modulating immunity against cancer. By understanding this neuro-immune axis, novel therapeutic approaches may be uncovered to strengthen antitumour immunity and enhance responses to existing cancer treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Zhao, L., Singh, V., Ricca, A. & Lee, P. Survival benefit of pembrolizumab for patients with pancreatic adenocarcinoma: a case series. J. Med. Cases 13, 240–243 (2022).
Storandt, M. H., Tran, N., Martin, N. & Jatoi, A. Pembrolizumab near the end of life in patients with metastatic pancreatic cancer: a multi-site consecutive series to examine survival and patient treatment burden. Cancer Immunol. Immunother. 72, 2515–2520 (2023).
Emens, L. A. et al. Challenges and opportunities in cancer immunotherapy: a Society for Immunotherapy of Cancer (SITC) strategic vision. J. Immunother. Cancer 12, e009063 (2024).
Young, H. H. On the presence of nerves in tumors and of other structures in them as revealed by a modification of Ehrlich’s method of ‘vital staining’ with methylene blue. J. Exp. Med. 2, 1–12 (1897).
Baraldi, J. H., Martyn, G. V., Shurin, G. V. & Shurin, M. R. Tumor innervation: history, methodologies, and significance. Cancers 14, 1979 (2022).
Lucido, C. T. et al. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 154, 228–235 (2019).
Zhao, Q. et al. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer 14, 484 (2014). This study provides early evidence suggesting that tumour denervation modulates tumour growth.
Ayala, G. E. et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 14, 7593–7603 (2008). This seminal study serves as an early example of human tumour innervation, identifying semaphorin 4F as a molecular driver of this process.
Entschladen, F., Palm, D., Lang, K., Drell, T. L. & Zaenker, K. S. Neoneurogenesis: tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med. Hypotheses 67, 33–35 (2006).
Madeo, M. et al. Cancer exosomes induce tumor innervation. Nat. Commun. 9, 4284 (2018). This article highlights cancer-derived exosomes as a driver of tumour innervation.
Amit, M. et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578, 449–454 (2020).
Mauffrey, P. et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569, 672–678 (2019).
Dobrenis, K., Gauthier, L. R., Barroca, V. & Magnon, C. Granulocyte colony‐stimulating factor off‐target effect on nerve outgrowth promotes prostate cancer development. Int. J. Cancer 136, 982–988 (2015).
Hanahan, D. & Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 41, 573–580 (2023).
Winkler, F. et al. Cancer neuroscience: state of the field, emerging directions. Cell 186, 1689–1707 (2023).
Magnon, C. & Hondermarck, H. The neural addiction of cancer. Nat. Rev. Cancer 23, 317–334 (2023).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Zhao, C.-M. et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 6, 250ra115 (2014).
Khanmammadova, N., Islam, S., Sharma, P. & Amit, M. Neuro-immune interactions and immuno-oncology. Trends Cancer 9, 636–649 (2023).
Saloman, J. L. et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl Acad. Sci. USA 113, 3078–3083 (2016). This work is the first demonstration that denervation of nociceptor neurons reduces tumour growth.
Erin, N. et al. Activation of neuroimmune pathways increases therapeutic effects of radiotherapy on poorly differentiated breast carcinoma. Brain. Behav. Immun. 48, 174–185 (2015).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013). This article presents the first demonstration that tumours are innervated and that this innervation controls tumour growth.
Renz, B. W. et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33, 75–90.e7 (2018).
Partecke, L. I. et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFα in a murine pancreatic cancer model. Oncotarget 8, 22501–22512 (2017).
Sampson, J. H., Gunn, M. D., Fecci, P. E. & Ashley, D. M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 20, 12–25 (2020).
Strickland, M. R., Alvarez-Breckenridge, C., Gainor, J. F. & Brastianos, P. K. Tumor immune microenvironment of brain metastases: toward unlocking antitumor immunity. Cancer Discov. 12, 1199–1216 (2022).
Basbaum, A. I. & Julius, D. Toward better pain control. Sci. Am. 294, 60–67 (2006).
Julius, D. & Basbaum, A. I. Molecular mechanisms of nociception. Nature 413, 203–210 (2001).
Caterina, M. J. & Julius, D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517 (2001).
Story, G. M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003).
Foster, S. L., Talbot, S. & Woolf, C. J. CNS injury: IL-33 sounds the alarm. Immunity 42, 403–405 (2015).
Li, C.-L. et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 26, 83–102 (2016).
Kupari, J. et al. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat. Commun. 12, 1510 (2021).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143.e23 (2019).
Kupari, J., Häring, M., Agirre, E., Castelo-Branco, G. & Ernfors, P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 27, 2508–2523.e4 (2019).
Kim, S.-H. et al. Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for pirt, TRPV1, 5-HT3, and Tac1 expression. eNeuro https://doi.org/10.1523/ENEURO.0494-19.2020 (2020).
Tochitsky, I. et al. Inhibition of inflammatory pain and cough by a novel charged sodium channel blocker. Br. J. Pharmacol. 178, 3905–3923 (2021).
Mathur, S. et al. Nociceptor neurons promote IgE class switch in B cells. JCI Insight 6, e148510 (2021).
Chiu, I. M., von Hehn, C. A. & Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067 (2012).
Bhuiyan, S. A. et al. Harmonized cross-species cell atlases of trigeminal and dorsal root ganglia. Sci. Adv. 10, eadj9173 (2024).
Jain, A. et al. Nociceptor-immune interactomes reveal insult-specific immune signatures of pain. Nat. Immunol. 25, 1296–1305 (2024).
Renthal, W. et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes axonal injury. Neuron 108, 128–144.e9 (2020).
Crosson, T. et al. Profiling of how nociceptor neurons detect danger—new and old foes. J. Intern. Med. 286, 268–289 (2019).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Chiu, I. M. et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 3, e04660 (2014).
Chiu, I. M. et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57 (2013). This important article demonstrates how CGRP released by nociceptor neurons controls antibacterial immunity.
Cohen, J. A. et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–932.e14 (2019).
Tamari, M. et al. Sensory neurons promote immune homeostasis in the lung. Cell 187, 44–61.e17 (2024).
Baral, P. et al. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 24, 417–426 (2018).
Pinho-Ribeiro, F. A. et al. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 173, 1083–1097.e22 (2018).
Pinho-Ribeiro, F. A. et al. Bacteria hijack a meningeal neuroimmune axis to facilitate brain invasion. Nature 615, 472–481 (2023).
Gao, Z. et al. Nociceptor neurons are involved in the host response to Escherichia coli urinary tract infections. J. Inflamm. Res. 15, 3337–3353 (2022).
Yang, N. J. et al. Nociceptive sensory neurons mediate inflammation induced by Bacillus anthracis edema toxin. Front. Immunol. 12, 642373 (2021).
Enamorado, M. et al. Immunity to the microbiota promotes sensory neuron regeneration. Cell 186, 607–620.e17 (2023).
Defaye, M. et al. Gut-innervating TRPV1+ neurons drive chronic visceral pain via microglial P2Y12 receptor. Cell. Mol. Gastroenterol. Hepatol. 13, 977–999 (2022).
Serger, E. et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 607, 585–592 (2022).
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Matheis, F. et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180, 64–78.e16 (2020).
Tränkner, D., Hahne, N., Sugino, K., Hoon, M. A. & Zuker, C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc. Natl Acad. Sci. USA 111, 11515–11520 (2014).
Klose, C. S. N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017).
Talbot, S., Foster, S. L. & Woolf, C. J. Neuroimmunity: physiology and pathology. Annu. Rev. Immunol. 34, 421–447 (2016).
Vats, K. et al. Sensory nerves impede the formation of tertiary lymphoid structures and development of protective antimelanoma immune responses. Cancer Immunol. Res. 10, 1141–1154 (2022).
Balood, M. et al. Nociceptor neurons affect cancer immunosurveillance. Nature 611, 405–412 (2022). This study provides evidence that CGRP regulates antitumour immune responses in melanoma.
Sinha, S. et al. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res. 77, 1868–1879 (2017).
Meerschaert, K. A. et al. Neuronally expressed PDL1, not PD1, suppresses acute nociception. Brain. Behav. Immun. 106, 233–246 (2022).
Wang, K. et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J. Clin. Invest. 130, 3603–3620 (2020).
Chen, G. et al. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat. Neurosci. 20, 917–926 (2017).
Zhang, H. et al. Intra-tumoural RAMP1+ B cells promote resistance to neoadjuvant anti-PD-1-based therapy in esophageal squamous cell carcinoma. Immunother. Adv. 5, ltaf012 (2025).
Tibensky, M. et al. Topical application of local anesthetics to melanoma increases the efficacy of anti-PD-1 therapy. Neoplasma 70, 375–389 (2023).
McIlvried, L. A., Atherton, M. A., Horan, N. L., Goch, T. N. & Scheff, N. N. Sensory neurotransmitter calcitonin gene-related peptide modulates tumor growth and lymphocyte infiltration in oral squamous cell carcinoma. Adv. Biol. 6, e2200019 (2022). This study demonstrates that CGRP regulates antitumour immune responses in head and neck cancers.
Darragh, L. B. et al. Sensory nerve release of CGRP increases tumor growth in HNSCC by suppressing TILs. Med 5, 254–270.e8 (2024).
Jiang, L. et al. Nociceptive adenosine A2A receptor on trigeminal nerves orchestrates CGRP release to regulate the progression of oral squamous cell carcinoma. Int. J. Oral. Sci. 16, 46 (2024).
Wu, V. H. et al. The GPCR–Gαs–PKA signaling axis promotes T cell dysfunction and cancer immunotherapy failure. Nat. Immunol. 24, 1318–1330 (2023).
Zhi, X. et al. Nociceptive neurons promote gastric tumour progression via a CGRP–RAMP1 axis. Nature 640, 802–810 (2025). This article highlights the presence of active neuro-neoplastic synapses in peripheral tumours and demonstrates that this communication is mediated by the CGRP–RAMP1 axis.
Wang, K. et al. Nociceptor neurons promote PDAC progression and cancer pain by interaction with cancer-associated fibroblasts and suppression of natural killer cells. Cell Res. 35, 362–380 (2025).
Zhang, Y. et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 34, 1999–2017.e10 (2022).
Islam, S. et al. Neural landscape is associated with functional outcomes in irradiated patients with oropharyngeal squamous cell carcinoma. Sci. Transl. Med. 16, eabq5585 (2024).
Suvas, S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J. Immunol. 199, 1543–1552 (2017).
Singh, S. et al. Neuropeptide substance P enhances inflammation-mediated tumor signaling pathways and migration and proliferation of head and neck cancers. Indian. J. Surg. Oncol. 12, 93–102 (2021).
Bencze, N. et al. Desensitization of capsaicin-sensitive afferents accelerates early tumor growth via increased vascular leakage in a murine model of triple negative breast cancer. Front. Oncol. 11, 685297 (2021).
Restaino, A. C. et al. Functional neuronal circuits promote disease progression in cancer. Sci. Adv. 9, eade4443 (2023).
Padmanaban, V. et al. Neuronal substance P drives metastasis through an extracellular RNA–TLR7 axis. Nature 633, 207–215 (2024). This article shows that substance P, produced by nociceptor neurons, modulates tumour growth.
Ravindranathan, S. et al. Targeting vasoactive intestinal peptide-mediated signaling enhances response to immune checkpoint therapy in pancreatic ductal adenocarcinoma. Nat. Commun. 13, 6418 (2022).
Kandel, E. R., Koester, J. D., Mack, S. H. & Siegelbaum, S. A. Principles of Neural Science 6th edn (McGraw Hill, 2021).
Karemaker, J. M. An introduction into autonomic nervous function. Physiol. Meas. 38, R89–R118 (2017).
Wehrwein, E. A., Orer, H. S. & Barman, S. M. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr. Physiol. 6, 1239–1278 (2016).
McCorry, L. K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 71, 78 (2007).
Berthoud, H. R. & Neuhuber, W. L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85, 1–17 (2000).
Atsumi, K. et al. Sensory neurons in the human jugular ganglion. Tissue Cell 64, 101344 (2020).
Bauer, K. C. et al. The gut microbiome controls liver tumors via the vagus nerve. Preprint at bioRxiv https://doi.org/10.1101/2024.01.23.576951 (2024).
Renz, B. W. et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8, 1458–1473 (2018).
Zheng, C. et al. Tumor-specific cholinergic CD4+ T lymphocytes guide immunosurveillance of hepatocellular carcinoma. Nat. Cancer 4, 1437–1454 (2023).
Zhu, J. et al. Tumour immune rejection triggered by activation of α2-adrenergic receptors. Nature 618, 607–615 (2023). This article demonstrates that clonidine-mediated α2-AR activation promotes tumour elimination.
Nevin, J. T., Moussa, M., Corwin, W. L., Mandoiu, I. I. & Srivastava, P. K. Sympathetic nervous tone limits the development of myeloid-derived suppressor cells. Sci. Immunol. 5, eaay9368 (2020).
Rump, L. C., Ruff, G., Wolk, V. & Schollmeyer, P. α2-Adrenoceptor activation inhibits noradrenaline release in human and rabbit isolated renal arteries. Eur. J. Pharmacol. 196, 277–283 (1991).
Sloan, E. K. et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 (2010).
Le, C. P. et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 7, 10634 (2016).
Graham, J. A. et al. Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3β inhibition. J. Biol. Chem. 285, 32852–32859 (2010).
Globig, A.-M. et al. The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature 622, 383–392 (2023). This article shows that adrenergic neurons promote T cell exhaustion through the β1-AR.
Fu, S. et al. Regulatory mucosa-associated invariant T cells controlled by β1 adrenergic receptor signaling contribute to hepatocellular carcinoma progression. Hepatology 78, 72–87 (2023).
Mohammadpour, H. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest. 129, 5537–5552 (2019).
Mohammadpour, H., MacDonald, C. R., McCarthy, P. L., Abrams, S. I. & Repasky, E. A. β2-Adrenergic receptor signaling regulates metabolic pathways critical to myeloid-derived suppressor cell function within the TME. Cell Rep. 37, 109883 (2021).
Daneshmandi, S. et al. Myeloid-derived suppressor cell mitochondrial fitness governs chemotherapeutic efficacy in hematologic malignancies. Nat. Commun. 15, 2803 (2024).
Xu, Q. et al. Multiple cancer cell types release LIF and Gal3 to hijack neural signals. Cell Res. 34, 345–354 (2024).
Qiao, G. et al. β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. 68, 11–22 (2019).
Qiao, G. et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol. Res. 9, 651–664 (2021).
Bucsek, M. J. et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 77, 5639–5651 (2017).
Devi, S. et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230.e7 (2021).
Chen, Z., Han, F., Du, Y., Shi, H. & Zhou, W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal. Transduct. Target. Ther. 8, 70 (2023).
Kokolus, K. M. et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7, e1405205 (2018).
Gupta, S. et al. Navigating the blurred path of mixed neuro-immune signaling. J. Allergy Clin. Immunol. 153, 924–938 (2024).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Fujii, T. et al. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 134, 1–21 (2017).
Zhang, B. et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature 599, 471–476 (2021).
Loeza-Alcocer, E., McPherson, T. P. & Gold, M. S. Peripheral GABA receptors regulate colonic afferent excitability and visceral nociception. J. Physiol. 597, 3425–3439 (2019).
Schneider, M. A. et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci. Transl. Med. 13, eabc8188 (2021).
Chen, Y. et al. Dopamine signaling promotes tissue-resident memory differentiation of CD8+ T cells and antitumor immunity. Cancer Res. 82, 3130–3142 (2022).
Wu, Y. et al. Dopamine uses the DRD5–ARRB2–PP2A signaling axis to block the TRAF6-mediated NF-κB pathway and suppress systemic inflammation. Mol. Cell 78, 42–56.e6 (2020).
Martyn, G. V., Shurin, G. V., Keskinov, A. A., Bunimovich, Y. L. & Shurin, M. R. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol. Immunother. 68, 1819–1829 (2019).
Rangel‐Sosa, M. M., Mann, F. & Chauvet, S. Pancreatic Schwann cell reprogramming supports cancer‐associated neuronal remodeling. Glia 72, 1840–1861 (2024).
Kruglov, O. et al. Melanoma-associated repair-like Schwann cells suppress anti-tumor T-cells via 12/15-LOX/COX2-associated eicosanoid production. Oncoimmunology 12, 2192098 (2023).
Zhang, S., Chen, J., Cheng, F. & Zheng, F. The emerging role of Schwann cells in the tumor immune microenvironment and its potential clinical application. Int. J. Mol. Sci. 25, 13722 (2024).
Shurin, M. R., Wheeler, S. E., Shurin, G. V., Zhong, H. & Zhou, Y. Schwann cells in the normal and pathological lung microenvironment. Front. Mol. Biosci. 11, 1365760 (2024).
Berner, J. et al. Human repair‐related Schwann cells adopt functions of antigen‐presenting cells in vitro. Glia 70, 2361–2377 (2022).
Xue, M. et al. Schwann cells regulate tumor cells and cancer-associated fibroblasts in the pancreatic ductal adenocarcinoma microenvironment. Nat. Commun. 14, 4600 (2023).
Duarte Mendes, A. et al. β-Adrenergic blockade in advanced non-small cell lung cancer patients receiving immunotherapy: a multicentric study. Cureus 16, e52194 (2024).
Gandhi, S. et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin. Cancer Res. 27, 87–95 (2021).
Mellgard, G. et al. Effect of concurrent β-blocker use in patients receiving immune checkpoint inhibitors for advanced solid tumors. J. Cancer Res. Clin. Oncol. 149, 2833–2841 (2023).
Oh, M. S. et al. The impact of β blockers on survival outcomes in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin. Lung Cancer 22, e57–e62 (2021).
Cortellini, A. et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J. Immunother. Cancer 9, e002421 (2021).
Yan, X. et al. Novel evidence for the prognostic impact of β-blockers in solid cancer patients receiving immune checkpoint inhibitors. Int. Immunopharmacol. 113, 109383 (2022).
Kennedy, O. J. & Neary, M. T. Brief communication on the impact of β-blockers on outcomes in patients receiving cancer immunotherapy. J. Immunother. 45, 303–306 (2022).
Zhang, Y. et al. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and β-blockers on outcomes in patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Oncoimmunology 10, 1957605 (2021).
Gandhi, S. et al. Impact of concomitant medication use and immune-related adverse events on response to immune checkpoint inhibitors. Immunotherapy 12, 141–149 (2020).
Wu, Y. L. et al. Outcomes of β blocker use in advanced hepatocellular carcinoma treated with immune checkpoint inhibitors. Front. Oncol. 13, 1128569 (2023).
Oren, O., Yang, E. H., Molina, J. R., Bailey, K. & Kopecky, S. β-Blocker use is associated with increased all-cause mortality in lung cancer patients receiving immune checkpoint inhibitors. J. Am. Coll. Cardiol. 75, 3519 (2020).
Chen, H. Y. et al. β-Blocker use is associated with worse relapse-free survival in patients with head and neck cancer. JCO Precis. Oncol. 7, e2200490 (2023).
Scheff, N. N. et al. Pretreatment pain predicts perineural invasion in patients with head and neck squamous cell carcinoma. Support. Care Cancer 31, 405 (2023).
Yaniv, D., Mattson, B., Talbot, S., Gleber-Netto, F. O. & Amit, M. Targeting the peripheral neural-tumour microenvironment for cancer therapy. Nat. Rev. Drug Discov. 23, 780–796 (2024).
Guillot, J. et al. Sympathetic axonal sprouting induces changes in macrophage populations and protects against pancreatic cancer. Nat. Commun. 13, 1985 (2022).
Goswami, P., Ives, A. M., Abbott, A. R. N. & Bertke, A. S. Stress hormones epinephrine and corticosterone selectively reactivate HSV-1 and HSV-2 in sympathetic and sensory neurons. Viruses 14, 1115 (2022).
Sloan, E. K., Tarara, R. P., Capitanio, J. P. & Cole, S. W. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J. Virol. 80, 4326–4335 (2006).
Sloan, E. K. et al. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J. Neurosci. 27, 8857–8865 (2007).
Brabenec, L., Gupta, S., Eichwald, T., Rafei, M. & Talbot, S. Decoding the neuroimmune axis in the atopic march: mechanisms and implications. Curr. Opin. Immunol. 91, 102507 (2024).
McNeil, B. D. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 (2015).
Serhan, N. et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 20, 1435–1443 (2019).
Green, D. P., Limjunyawong, N., Gour, N., Pundir, P. & Dong, X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101, 412–420.e3 (2019).
Caceres, A. I. et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl Acad. Sci. USA 106, 9099–9104 (2009).
Bautista, D. M., Pellegrino, M. & Tsunozaki, M. TRPA1: a gatekeeper for inflammation. Annu. Rev. Physiol. 75, 181–200 (2013).
Dunzendorfer, S., Meierhofer, C. & Wiedermann, C. J. Signaling in neuropeptide-induced migration of human eosinophils. J. Leukoc. Biol. 64, 828–834 (1998).
Talbot, S. et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87, 341–354 (2015).
Rochlitzer, S. et al. The neuropeptide calcitonin gene-related peptide affects allergic airway inflammation by modulating dendritic cell function. Clin. Exp. Allergy 41, 1609–1621 (2011).
Li, M. et al. Deficiency of RAMP1 attenuates antigen-induced airway hyperresponsiveness in mice. PLoS One 9, e102356 (2014).
Foster, S. L., Seehus, C. R., Woolf, C. J. & Talbot, S. Sense and immunity: context-dependent neuro-immune interplay. Front. Immunol. 8, 1463 (2017).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 (2017).
Yu, J. W. et al. Tumor-immune profiling of murine syngeneic tumor models as a framework to guide mechanistic studies and predict therapy response in distinct tumor microenvironments. PLoS One 13, e0206223 (2018).
Olive, K. P. & Politi, K. Translational therapeutics in genetically engineered mouse models of cancer. Cold Spring Harb. Protoc. 2014, 131–143 (2014).
Liu, Y. et al. Patient-derived xenograft models in cancer therapy: technologies and applications. Signal. Transduct. Target. Ther. 8, 160 (2023).
Perrin, P., Charroin, P. & Durand, L. Bladder tumors treated with radical cystectomy. Results of 78 cases [French]. J. Urol. 89, 243–246 (1983).
Chang, R. B. Optogenetic control of the peripheral nervous system. Cold Spring Harb. Perspect. Med. 9, a034397 (2019).
Kang, H. J. et al. Structure-guided design of a peripherally restricted chemogenetic system. Cell 187, 7433–7449.e20 (2024).
Iyer, S. M. et al. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci. Rep. 6, 30570 (2016).
Saleeba, C., Dempsey, B., Le, S., Goodchild, A. & McMullan, S. A student’s guide to neural circuit tracing. Front. Neurosci. 13, 897 (2019).
Thiel, V. et al. Characterization of single neurons reprogrammed by pancreatic cancer. Nature 640, 1042–1051 (2025). This work uses neuronal tracing and single-cell RNA-sequencing in combination to show how tumour-innervating neurons are reprogrammed by PDAC.
Binshtok, A. M., Bean, B. P. & Woolf, C. J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449, 607–610 (2007).
Talbot, S. et al. Vagal sensory neurons drive mucous cell metaplasia. J. Allergy Clin. Immunol. 145, 1693–1696.e4 (2020).
Roversi, K. et al. Nanophotonics enable targeted photothermal silencing of nociceptor neurons. Small 18, e2103364 (2022).
McDougall, J. J. & O’Brien, M. S. Analgesic potential of voltage gated sodium channel modulators for the management of pain. Curr. Opin. Pharmacol. 75, 102433 (2024).
Sapio, M. R. et al. Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. J. Clin. Invest. 128, 1657–1670 (2018).
Clyburn, C., Andresen, M. C., Ingram, S. L. & Habecker, B. A. Untangling peripheral sympathetic neurocircuits. Front. Cardiovasc. Med. 9, 842656 (2022).
Kingwell, K. NaV1.8 inhibitor poised to provide opioid-free pain relief. Nat. Rev. Drug. Discov. 24, 3–5 (2025).
Haykin, H. & Rolls, A. The neuroimmune response during stress: a physiological perspective. Immunity 54, 1933–1947 (2021).
Alotiby, A. Immunology of stress: a review article. J. Clin. Med. 13, 6394 (2024).
Freier, E. et al. Decrease of CD4+FOXP3+ T regulatory cells in the peripheral blood of human subjects undergoing a mental stressor. Psychoneuroendocrinology 35, 663–673 (2010).
Jürgens, M. et al. Chronic stimulation desensitizes β2‐adrenergic receptor responses in natural killer cells. Eur. J. Immunol. 54, e2451299 (2024).
Jean-Charles, P.-Y., Kaur, S. & Shenoy, S. K. G protein-coupled receptor signaling through β-arrestin-dependent mechanisms. J. Cardiovasc. Pharmacol. 70, 142–158 (2017).
Zhang, M. et al. G protein-coupled receptors (GPCRs): advances in structures, mechanisms and drug discovery. Signal. Transduct. Target. Ther. 9, 88 (2024).
Wei, X. et al. Myeloid β-arrestin 2 depletion attenuates metabolic dysfunction-associated steatohepatitis via the metabolic reprogramming of macrophages. Cell Metab. 36, 2281–2297.e7 (2024).
Qin, R. et al. β-Arrestin 1 promotes the progression of chronic myeloid leukaemia by regulating BCR/ABL H4 acetylation. Br. J. Cancer 111, 568–576 (2014).
Fereshteh, M. et al. β-Arrestin 2 mediates the initiation and progression of myeloid leukemia. Proc. Natl Acad. Sci. USA 109, 12532–12537 (2012).
Acknowledgements
M.A.’s work is supported by the National Institutes of Health (NIH) (National Cancer Institute (NCI), R37CA242006-01A1), the Stiefel Family Discovery Award, an Institutional Research Grant and the Disruptive Science Moonshot Award at MD Anderson Cancer Center (MDACC). A.C.’s work is supported by a National Health and Medical Research Council (NHMRC) grant (2020851). A.R. is supported by a scholarship from Fonds de recherche du Québec (FRQS) (347343). T.E. is supported by the Brain Canada Rising Star Program, funded by the Henry and Berenice Kaufmann Foundation. K.O.D. is supported by the Swiss National Science Foundation (TMSGI3_218400, PZ00P3_202029) and the NCI (R21CA282866). P.D.V.’s work is funded by the National Institute of Dental and Craniofacial Research (NIDCR) (5R01DE032712) and the National Institute of General Medical Sciences (NIGMS) (P30GM145398). N.N.S.’s work is supported by the Rita Allen Foundation Pain Award (2021) and the NIH/NIDCR (R01DE030892, R01DE033473, R21DE034106). S.T.’s work is supported by the Canadian Institutes of Health Research (CIHR) (193741, 407016, 461274, 461275), the Canada Foundation for Innovation (44135), the Canadian Cancer Society Emerging Scholar Research Grant (708096), the Knut and Alice Wallenberg Foundation (KAW 2021.0141, KAW 2022.0327), the Swedish Research Council (2022-01661), the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-06824) and the NIH/NIDCR (R01DE032712).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed to discussions on its content and drafted the manuscript under the guidance of S.T., N.N.S., and M.A. All authors reviewed and approved the final version before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Elizabeth Repasky who co-reviewed with Jee Eun Choi, Felipe A. Pinho-Ribeiro who co-reviewed with Tiago H. Zaninelli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- α7-Nicotinic acetylcholine receptors
-
(α7-nAChRs). Cholinergic receptors with high calcium permeability, strongly implicated in cognitive function, inflammation modulation and neuroprotection.
- β-Adrenergic receptors
-
(β-ARs). Protein-coupled receptors responsive to adrenaline and norepinephrine, regulating cardiac output, smooth muscle tone and metabolism.
- Acid-sensing ion channels
-
(ASICS). Proton-gated ion channels activated by acidic environments, contributing to pain detection and modulating neuronal excitability.
- Chemogenetic tools
-
A technique merging engineered receptors with selective ligands to modulate neuronal activity, enabling manipulation of signalling.
- Cranial nerves
-
Twelve nerves arising from the brain, controlling motor, sensory and parasympathetic functions in head and neck.
- Dorsal root ganglia
-
Clusters of sensory neuron bodies in spinal nerves, transmitting signals into the central nervous system.
- Enterochromaffin cells
-
Specialized cells within the gastrointestinal tract secreting serotonin, influencing gut motility, secretion and nervous signalling.
- Fight-or-flight responses
-
Physiological reactions triggered by threats, mediated by sympathetic activity, increasing alertness, energy and resource mobilization.
- Group 2 innate lymphoid cell
-
An immune cell producing type 2 cytokines, such as interleukin-5 (IL-5) and IL-13, orchestrating responses against parasites, regulating tissue repair and homeostasis.
- Humanized mice
-
Genetically modified mice expressing human genes, cells or tissues, for disease modelling and testing in vivo.
- Intermediolateral cell column
-
Lateral grey horn region of the spinal cord housing preganglionic sympathetic neurons for autonomic regulation.
- Muscarinic receptors
-
G-protein-coupled acetylcholine receptors modulate parasympathetic functions, including secretion, the heart rate and smooth muscle contraction.
- Muscularis macrophages
-
Specialized macrophages residing in the smooth muscle layer of the gastrointestinal tract that have crucial roles in modulating gut motility, maintaining tissue homeostasis and coordinating immune responses.
- Neurogenesis
-
Process generating new neurons from progenitor cells, primarily during development and in adult brain regions.
- Neuro-immune reflex
-
Reflexive interplay between neurons and immune cells, rapidly modulating inflammation and sustaining homeostasis through coordinated signals.
- Neurotrophins
-
Proteins supporting neuron survival, differentiation and plasticity, including nerve growth factors (NGFs) and brain-derived neurotrophic factors (BDNFs).
- Nodose and jugular ganglia
-
Sensory ganglia of the vagus nerve, housing afferent neurons regulating cardiovascular, respiratory and gastrointestinal reflexes.
- Optogenetic tools
-
A method using light-sensitive proteins to control neuronal activity with spatial and temporal resolution in vivo.
- Parasympathetic nervous system
-
(PNS). Division of the autonomic nervous system conserving energy, slowing the heart rate and promoting glandular activities.
- Paraventricular nucleus
-
A key hypothalamic region composed of distinct neuronal populations that integrate neural and hormonal signals to regulate autonomic functions, stress responses, fluid balance and energy homeostasis.
- Paravertebral ganglia
-
Sympathetic ganglia adjacent to the spinal column, transmitting signals for autonomic regulation of body functions.
- Perineural invasion
-
Cancer cells invading nerves, facilitating tumour spread, associated with pain, recurrence and poor outcomes.
- PIEZO channels
-
Mechanically activated ion channels sensing pressure, touch and stretch, crucial for mechanotransduction in various tissues.
- Prevertebral ganglia
-
Sympathetic ganglia located anterior to the vertebral column, regulating innervation of abdominal and pelvic viscera.
- Purinergic receptors
-
Receptors activated by extracellular nucleotides such as ATP, mediating diverse nociception, inflammation and cell death signalling.
- Rest-and-digest activities
-
Parasympathetic-driven processes conserving energy, supporting digestion, glandular secretions and facilitating overall recovery from sympathetic activation.
- Restraint stress
-
Stressful condition induced by restricting movement, triggering physiological and psychological responses, including elevated corticosterone levels.
- Second-order neuronal synapses
-
Central nervous system synapses receiving input from afferent neurons, relaying signals onwards to a higher centre.
- Splanchnic nerves
-
Visceral nerves carrying sympathetic and parasympathetic fibres, innervating abdominal and pelvic organs, modulating autonomic function.
- Sympathetic nervous system
-
(SNS). Division of the autonomic nervous system promoting fight-or-flight responses, increasing the heart rate and energy mobilization.
- Tertiary lymphoid structures
-
(TLS). Ectopic clusters of immune cells in non-lymphoid tissues, supporting immunity and antigen presentation during inflammation.
- Transient receptor potential (TRP) channels
-
Cation-permeable ion channels responsive to temperature, chemical and mechanical stimuli, for sensory transduction across modalities.
- Trigeminal ganglia
-
Sensory ganglia of the trigeminal nerve, conveying facial sensation, controlling motor functions for mastication and biting.
- Type 17 immune responses
-
Immunity mediated by interleukin-17 (IL-17)-producing cells, important for defence against extracellular pathogens and contributing to inflammation.
- Ventral roots
-
Neural outflows from the spinal cord, carrying efferent signals to skeletal muscles and autonomic ganglia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amit, M., Eichwald, T., Roger, A. et al. Neuro-immune cross-talk in cancer. Nat Rev Cancer (2025). https://doi.org/10.1038/s41568-025-00831-w
Accepted:
Published:
DOI: https://doi.org/10.1038/s41568-025-00831-w