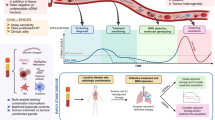
Abstract
Circulating tumour DNA (ctDNA) can be released by cancer cells into biological fluids through apoptosis, necrosis or active release. In patients with non-small-cell lung cancer (NSCLC), ctDNA levels correlate with clinical and pathological factors, including histology, tumour size and proliferative status. Currently, ctDNA analysis is recommended for molecular profiling in patients with advanced-stage NSCLC. In this Review, we summarize the increasing evidence suggesting that ctDNA has potential clinical applications in the management of patients with early stage and locally advanced NSCLC. In those with early stage NSCLC, detection of ctDNA before and/or after surgery is associated with a greater risk of disease recurrence. Longitudinal monitoring after surgery can further increase the prognostic value of ctDNA testing and enables detection of disease recurrence earlier than the assessment of clinical or radiological progression. In patients with locally advanced NSCLC, the detection of ctDNA after chemoradiotherapy is also associated with a greater risk of disease progression. Owing to the limited number of patients enrolled and the different technologies used for ctDNA testing in most of the clinical studies performed thus far, their results are not sufficient to currently support the routine clinical use of ctDNA monitoring in patients with early stage or locally advanced NSCLC. Therefore, we discuss the need for interventional studies to provide evidence for implementing ctDNA testing in this setting.
Key points
-
The availability of neoadjuvant and adjuvant treatments for patients with early stage non-small-cell lung cancer (NSCLC) makes it necessary to identify prognostic and predictive biomarkers to better personalize treatments.
-
Circulating tumour DNA (ctDNA) testing is currently recommended for biomarker analysis in patients with advanced-stage NSCLC. Increasing evidence suggests that ctDNA testing has several potential clinical applications in patients with early stage disease, although this evidence mostly comes from retrospective analyses using assays with different sensitivity and specificity and often in small patient cohorts.
-
In patients with early stage NSCLC, the detection of ctDNA before surgery is associated with a significantly higher risk of relapse.
-
In patients receiving neoadjuvant therapy, the dynamics of ctDNA clearance correlate with pathological response and prognosis.
-
The detection of minimal residual disease by ctDNA testing after surgery or definitive chemoradiotherapy correlates with a significantly higher risk of relapse.
-
The incorporation of ctDNA-based assays in the management of patients with early stage NSCLC will require standardization of tests (with definition of a minimum threshold for sensitivity and specificity) and prospective clinical trials in which patients are stratified based on ctDNA testing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Remon, J., Soria, J. C., Peters, S. & ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 32, 1637–1642 (2021).
Pignon, J. P. et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 26, 3552–3559 (2008).
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet 398, 535–554 (2021).
Saw, S. P. L., Ong, B. H., Chua, K. L. M., Takano, A. & Tan, D. S. W. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol. 22, e501–e516 (2021).
Cascone, T. et al. Perioperative nivolumab in resectable lung cancer. N. Engl. J. Med. 390, 1756–1769 (2024).
Pascual, J. et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 33, 750–768 (2022).
Mosele, F. et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 31, 1491–1505 (2020).
Hendriks, L. E. et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34, 339–357 (2023).
Mack, P. C. et al. Spectrum of driver mutations and clinical impact of circulating tumor DNA analysis in non-small cell lung cancer: analysis of over 8000 cases. Cancer 126, 3219–3228 (2020).
Roma, C. et al. Low impact of clonal hematopoiesis on the determination of RAS mutations by cell-free DNA testing in routine clinical diagnostics. Diagnostics 12, 1956 (2022).
Aggarwal, C. et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell lung cancer. JAMA Oncol. 5, 173–180 (2019).
Pasquale, R. et al. Targeted sequencing analysis of cell-free DNA from metastatic non-small-cell lung cancer patients: clinical and biological implications. Transl. Lung Cancer Res. 9, 61–70 (2020).
Leighl, N. B. et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin. Cancer Res. 25, 4691–4700 (2019).
Schwaederle, M. C. et al. Utility of genomic assessment of blood-derived circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin. Cancer Res. 23, 5101–5111 (2017).
Remon, J. et al. Outcomes in oncogenic-addicted advanced NSCLC patients with actionable mutations identified by liquid biopsy genomic profiling using a tagged amplicon-based NGS assay. PLoS ONE 15, e0234302 (2020).
Papadopoulou, E. et al. Clinical feasibility of NGS liquid biopsy analysis in NSCLC patients. PLoS ONE 14, e0226853 (2019).
Russo, A. et al. Liquid biopsy of lung cancer before pathological diagnosis is associated with shorter time to treatment. JCO Precis. Oncol. 8, e2300535 (2024).
Normanno, N. et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann. Oncol. 29, 112–118 (2018).
Bestvina, C. M. et al. Early-stage lung cancer: using circulating tumor DNA to get personal. J. Clin. Oncol. 41, 4093–4096 (2023).
Nagasaka, M. et al. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer. Mol. Cancer 20, 82 (2021).
Cohen, S. A., Liu, M. C. & Aleshin, A. Practical recommendations for using ctDNA in clinical decision making. Nature 619, 259–268 (2023).
Normanno, N., Cervantes, A., Ciardiello, F., De Luca, A. & Pinto, C. The liquid biopsy in the management of colorectal cancer patients: current applications and future scenarios. Cancer Treat. Rev. 70, 1–8 (2018).
Ignatiadis, M., Sledge, G. W. & Jeffrey, S. S. Liquid biopsy enters the clinic — implementation issues and future challenges. Nat. Rev. Clin. Oncol. 18, 297–312 (2021).
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Dasari, A. et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal-Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 17, 757–770 (2020).
Pantel, K. & Alix-Panabieres, C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat. Rev. Clin. Oncol. 16, 409–424 (2019).
Pellini, B. & Chaudhuri, A. A. Circulating tumor DNA minimal residual disease detection of non-small-cell lung cancer treated with curative intent. J. Clin. Oncol. 40, 567–575 (2022).
Moding, E. J., Nabet, B. Y., Alizadeh, A. A. & Diehn, M. Detecting liquid remnants of solid tumors: circulating tumor DNA minimal residual disease. Cancer Discov. 11, 2968–2986 (2021).
Anagnostou, V. & Velculescu, V. E. Pushing the boundaries of liquid biopsies for early precision intervention. Cancer Discov. 14, 615–619 (2024).
Esposito Abate, R. et al. Next generation sequencing-based profiling of cell free DNA in patients with advanced non-small cell lung cancer: advantages and pitfalls. Cancers 12, 3804 (2020).
Corcoran, R. B. & Chabner, B. A. Application of cell-free DNA analysis to cancer treatment. N. Engl. J. Med. 379, 1754–1765 (2018).
Stejskal, P. et al. Circulating tumor nucleic acids: biology, release mechanisms, and clinical relevance. Mol. Cancer 22, 15 (2023).
Diaz, L. A. Jr. & Bardelli, A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 32, 579–586 (2014).
Mattox, A. K. et al. The origin of highly elevated cell-free DNA in healthy individuals and patients with pancreatic, colorectal, lung, or ovarian cancer. Cancer Discov. 13, 2166–2179 (2023).
Mouliere, F. & Rosenfeld, N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proc. Natl Acad. Sci. USA 112, 3178–3179 (2015).
Mouliere, F. et al. High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE 6, e23418 (2011).
Heitzer, E., Haque, I. S., Roberts, C. E. S. & Speicher, M. R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20, 71–88 (2019).
Keller, L., Belloum, Y., Wikman, H. & Pantel, K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br. J. Cancer 124, 345–358 (2021).
Sanchez, C., Snyder, M. W., Tanos, R., Shendure, J. & Thierry, A. R. New insights into structural features and optimal detection of circulating tumor DNA determined by single-strand DNA analysis. NPJ Genom. Med. 3, 31 (2018).
El Messaoudi, S., Rolet, F., Mouliere, F. & Thierry, A. R. Circulating cell free DNA: preanalytical considerations. Clin. Chim. Acta 424, 222–230 (2013).
Han, D. S. C. & Lo, Y. M. D. The nexus of cfDNA and nuclease biology. Trends Genet. 37, 758–770 (2021).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra224 (2014).
Abbosh, C. et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545, 446–451 (2017).
Abbosh, C. et al. Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 616, 553–562 (2023).
Qiu, B. et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat. Commun. 12, 6770 (2021).
Santonja, A. et al. Comparison of tumor-informed and tumor-naive sequencing assays for ctDNA detection in breast cancer. EMBO Mol. Med. 15, e16505 (2023).
Kasi, P. M. Tumor-informed versus plasma-only liquid biopsy assay in a patient with multiple primary malignancies. JCO Precis. Oncol. 6, e2100298 (2022).
Black, J. et al. LBA55. An ultra-sensitive and specific ctDNA assay provides novel pre-operative disease stratification in early stage lung cancer. Ann. Oncol. 34, S1294 (2023).
Razavi, P. et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 25, 1928–1937 (2019).
Lo, Y. M. D., Han, D. S. C., Jiang, P. & Chiu, R. W. K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 372, eaaw3616 (2021).
Cristiano, S. et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 570, 385–389 (2019).
Bruhm, D. C. et al. Single-molecule genome-wide mutation profiles of cell-free DNA for non-invasive detection of cancer. Nat. Genet. 55, 1301–1310 (2023).
Annapragada, A. V. et al. Genome-wide repeat landscapes in cancer and cell-free DNA. Sci. Transl. Med. 16, eadj9283 (2024).
Mazzone, P. J. et al. Clinical validation of a cell-free DNA fragmentome assay for augmentation of lung cancer early detection. Cancer Discov. 14, 2224–2242 (2024).
Li, W. et al. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 21, 25 (2022).
Vandekerckhove, O. et al. Liquid biopsy in early-stage lung cancer: current and future clinical applications. Cancers 15, 2702 (2023).
Peng, M. et al. Circulating tumor DNA as a prognostic biomarker in localized non-small cell lung cancer. Front. Oncol. 10, 561598 (2020).
Xia, L. et al. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1). Clin. Cancer Res. 28, 3308–3317 (2022).
Li, N. et al. Perioperative circulating tumor DNA as a potential prognostic marker for operable stage I to IIIA non-small cell lung cancer. Cancer 128, 708–718 (2022).
Chabon, J. J. et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 580, 245–251 (2020).
Gale, D. et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann. Oncol. 33, 500–510 (2022).
Tan, A. C. et al. Detection of circulating tumor DNA with ultradeep sequencing of plasma cell-free DNA for monitoring minimal residual disease and early detection of recurrence in early-stage lung cancer. Cancer 130, 1758–1765 (2024).
Provencio, M. et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-small-cell lung cancer (NADIM phase II trial). J. Clin. Oncol. 40, 2924–2933 (2022).
Provencio, M. et al. Perioperative chemotherapy and nivolumab in non-small-cell lung cancer (NADIM): 5-year clinical outcomes from a multicentre, single-arm, phase 2 trial. Lancet Oncol. 25, 1453–1464 (2024).
Kris, M. G. et al. Dynamic circulating tumour DNA (ctDNA) response to neoadjuvant (NA) atezolizumab (atezo) and surgery (surg) and association with outcomes in patients (pts) with NSCLC. Ann. Oncol. 32, S1373 (2021).
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Deutsch, J. S. et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat. Med. 30, 218–228 (2024).
Reck, M. et al. Associations of ctDNA clearance (CL) during neoadjuvant Tx with pathological response and event-free survival (EFS) in pts with resectable NSCLC (R-NSCLC): expanded analyses from AEGEAN. Ann. Oncol. 35, S1239 (2024).
Nie, R. et al. Predictive value of radiological response, pathological response and relapse-free survival for overall survival in neoadjuvant immunotherapy trials: pooled analysis of 29 clinical trials. Eur. J. Cancer 186, 211–221 (2023).
Kelly, R. J. et al. Neoadjuvant nivolumab or nivolumab plus LAG-3 inhibitor relatlimab in resectable esophageal/gastroesophageal junction cancer: a phase Ib trial and ctDNA analyses. Nat. Med. 30, 1023–1034 (2024).
Jung, H. A. et al. Longitudinal monitoring of circulating tumor DNA from plasma in patients with curative resected stages I to IIIA EGFR-mutant non-small cell lung cancer. J. Thorac. Oncol. 18, 1199–1208 (2023).
John, T. et al. Molecular residual disease (MRD) analysis from the ADAURA trial of adjuvant (adj) osimertinib in patients (pts) with resected EGFR-mutated (EGFRm) stage IB–IIIA non-small cell lung cancer (NSCLC). J. Clin. Oncol. 42, https://doi.org/10.1200/JCO.2024.42.16_suppl.800 (2024).
Chen, K. et al. Individualized tumor-informed circulating tumor DNA analysis for postoperative monitoring of non-small cell lung cancer. Cancer Cell 41, 1749–1762.e6 (2023).
Zhang, J. T. et al. Longitudinal undetectable molecular residual disease defines potentially cured population in localized non-small cell lung cancer. Cancer Discov. 12, 1690–1701 (2022).
Zviran, A. et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat. Med. 26, 1114–1124 (2020).
Chaudhuri, A. A. et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 7, 1394–1403 (2017).
Kuang, P. P. et al. Circulating tumor DNA analyses as a potential marker of recurrence and effectiveness of adjuvant chemotherapy for resected non-small-cell lung cancer. Front. Oncol. 10, 595650 (2021).
Wang, S. et al. Circulating tumor DNA integrating tissue clonality detects minimal residual disease in resectable non-small-cell lung cancer. J. Hematol. Oncol. 15, 137 (2022).
Chen, K. et al. Perioperative dynamic changes in circulating tumor DNA in patients with lung cancer (DYNAMIC). Clin. Cancer Res. 25, 7058–7067 (2019).
Zhou, C. et al. IMpower010: biomarkers of disease-free survival (DFS) in a phase III study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLCIMpower010: biomarkers of disease-free survival (DFS) in a phase III study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLC. Ann. Oncol. https://doi.org/10.1016/j.annonc.2021.10.018 (2021).
Wu, Y. L. et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 383, 1711–1723 (2020).
Herbst, R. S. et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J. Clin. Oncol. 41, 1830–1840 (2023).
Felip, E. et al. IMpower010: ctDNA status in patients (pts) with resected NSCLC who received adjuvant chemotherapy (chemo) followed by atezolizumab (atezo) or best supportive care (BSC). Ann. Oncol. https://doi.org/10.1016/j.iotech.2022.100106 (2022).
Antonia, S. J. et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 379, 2342–2350 (2018).
Moding, E. J. et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat. Cancer 1, 176–183 (2020).
Yang, Y. et al. The clinical utility of dynamic ctDNA monitoring in inoperable localized NSCLC patients. Mol. Cancer 21, 117 (2022).
Pan, Y. et al. Dynamic circulating tumor DNA during chemoradiotherapy predicts clinical outcomes for locally advanced non-small cell lung cancer patients. Cancer Cell 41, 1763–1773.e4 (2023).
US Food and Drug Administration. FDA guidance document. Use of circulating tumor deoxyribonucleic acid for early-stage solid tumor drug development; draft guidance for industry; availability. FDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-circulating-tumor-deoxyribonucleic-acid-early-stage-solid-tumor-drug-development-guidance (2024).
Jamshidi, A. et al. Evaluation of cell-free DNA approaches for multi-cancer early detection. Cancer Cell 40, 1537–1549.e12 (2022).
van der Pol, Y. & Mouliere, F. Toward the early detection of cancer by decoding the epigenetic and environmental fingerprints of cell-free DNA. Cancer Cell 36, 350–368 (2019).
Foda, Z. H. et al. Detecting liver cancer using cell-free DNA fragmentomes. Cancer Discov. 13, 616–631 (2023).
Kim, S. Y. et al. Cancer signature ensemble integrating cfDNA methylation, copy number, and fragmentation facilitates multi-cancer early detection. Exp. Mol. Med. 55, 2445–2460 (2023).
Chung, D. C. et al. A cell-free DNA blood-based test for colorectal cancer screening. N. Engl. J. Med. 390, 973–983 (2024).
Bessa, X. et al. High accuracy of a blood ctDNA-based multimodal test to detect colorectal cancer. Ann. Oncol. 34, 1187–1193 (2023).
Sujit, S. J. et al. Enhancing NSCLC recurrence prediction with PET/CT habitat imaging, ctDNA, and integrative radiogenomics-blood insights. Nat. Commun. 15, 3152 (2024).
Moser, T., Kuhberger, S., Lazzeri, I., Vlachos, G. & Heitzer, E. Bridging biological cfDNA features and machine learning approaches. Trends Genet. 39, 285–307 (2023).
Forshew, T. et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 4, 136ra168 (2012).
Newman, A. M. et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat. Biotechnol. 34, 547–555 (2016).
Deveson, I. W. et al. Evaluating the analytical validity of circulating tumor DNA sequencing assays for precision oncology. Nat. Biotechnol. 39, 1115–1128 (2021).
Watson, C. J. et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367, 1449–1454 (2020).
Curtis, C. Quantifying mutations in healthy blood. Science 367, 1426–1427 (2020).
Martin-Alonso, C. et al. Priming agents transiently reduce the clearance of cell-free DNA to improve liquid biopsies. Science 383, eadf2341 (2024).
Tie, J. et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N. Engl. J. Med. 386, 2261–2272 (2022).
Tie, J. et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer: overall survival and updated 5-year results from the randomized DYNAMIC trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.2024.42.16_suppl.108 (2024).
Normanno, N. et al. Access and quality of biomarker testing for precision oncology in Europe. Eur. J. Cancer 176, 70–77 (2022).
Smeltzer, M. P. et al. The International Association for the Study of Lung Cancer global survey on molecular testing in lung cancer. J. Thorac. Oncol. 15, 1434–1448 (2020).
van Krieken, J. H. et al. Guideline on the requirements of external quality assessment programs in molecular pathology. Virchows Arch. 462, 27–37 (2013).
Fairley, J. A. et al. Implementation of circulating tumour DNA multi-target mutation testing in plasma: a perspective from an external quality assessment providers’ survey. Virchows Arch. 485, 717–722 (2023).
Waldeck, S. et al. Early assessment of circulating tumor DNA after curative-intent resection predicts tumor recurrence in early-stage and locally advanced non-small-cell lung cancer. Mol. Oncol. 16, 527–537 (2022).
Acknowledgements
N.N. and A.D.L. have received support from the Italian Ministry of Health (Project T3-AN-06 “Sviluppo di una piattaforma per la implementazione clinica della oncologia di precisione nelle regioni del centro-sud Italia” (N.N.) and Ricerca Corrente L3/7 (A.D.L.). E.B. currently receives support from the Associazione Italiana per la Ricerca sul Cancro (AIRC) under Investigator Grant Number IG20583, from the Ministero della Salute (Ricerca Corrente 2022-2023) and from the Università Cattolica del Sacro Cuore through institutional funds (UCSC-Project D1).
Author information
Authors and Affiliations
Contributions
N.N. researched data for the article. N.N. and A.D.L. wrote the article. A.M., A.M.R., V.S., L.L., E.B., A.D. and F.C. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.N. has received fees as a speaker and/or advisory board member from AstraZeneca, Bayer, Biocartis, Bristol Myers Squibb, Eli Lilly, Illumina, Incyte, Merck, Merck Sharpe & Dohme, Novartis, Roche, Sanofi and Thermofisher; works in an institution that receives financial support from AstraZeneca, Biocartis, Illumina, Merck, QIAGEN, Roche and Thermofisher; is the President of the International Quality Network for Pathology; and is the past President of the Italian Cancer Society. A.M. has received fees for lectures, presentations, speakers’ bureaus and/or educational events from AstraZeneca, Bristol Myers Squibb, Boehringer, Italfarmaco, Eli Lilly, Merck Sharpe & Dohme, Novartis, Pfizer, Roche, Sanofi and Takeda; and has been an advisory board member for AstraZeneca, Bristol Myers Squibb, Merck Sharpe & Dohme, Pfizer, Roche and Takeda. E.B. works in an institution that receives research grants from AstraZeneca and Roche; has received fees as a speaker and/or for travel from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Novartis, Pfizer and Roche; has received financial support as a speaker from Takeda Oncology; and serves on the Data Safety and Monitoring Board of Merck Sharpe & Dohme for activities outside of the submitted work. F.C. has received fees as an advisory board member and/or for lectures from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Galecto, Illumina, Eli Lilly, Merck Sharpe & Dohme, Mirati, Novocure, Ose Immunotherapeutics, Pfizer, Pharmamar, Roche, Sanofi, Takeda and Thermofisher. A.M.R., V.S., L.L., A.D. and A.D.L. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks L. Mezquita and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Normanno, N., Morabito, A., Rachiglio, A.M. et al. Circulating tumour DNA in early stage and locally advanced NSCLC: ready for clinical implementation?. Nat Rev Clin Oncol 22, 215–231 (2025). https://doi.org/10.1038/s41571-024-00985-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-024-00985-w