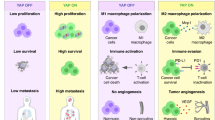
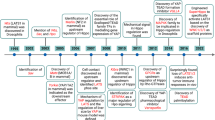
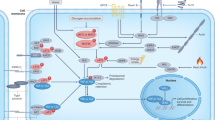
Abstract
The Hippo pathway is a highly conserved signalling network that controls tissue growth and cell fate, responding to physical properties of the tissue microenvironment and cell biological features such as adhesion and polarity. Hippo signalling perturbation is associated with several human diseases, particularly various solid cancers. Hippo pathway-targeted therapies are beginning to emerge for the treatment of cancer, most of which are focused on disrupting the ability of the YAP and TAZ transcription co-activator proteins to promote transcription of genes with their cognate TEAD1–4 DNA binding proteins. Recently, TEAD inhibitors have shown promise in a phase I clinical trial in cancers that are enriched for Hippo pathway mutations, such as mesothelioma. Moreover, Hippo pathway-targeted therapies have great potential to be combined with RAS–MAPK pathway inhibitors, given the close functional relationship that these signalling pathways share in development and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tapon, N. et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 (2002).
Pantalacci, S., Tapon, N. & Leopold, P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5, 921–927 (2003).
Justice, R. W., Zilian, O., Woods, D. F., Noll, M. & Bryant, P. J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 (1995).
Xu, T., Wang, W., Zhang, S., Stewart, R. A. & Yu, W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 (1995).
Wu, S., Huang, J., Dong, J. & Pan, D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with Salvador and Warts. Cell 114, 445–456 (2003).
Jia, J., Zhang, W., Wang, B., Trinko, R. & Jiang, J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17, 2514–2519 (2003).
Kango-Singh, M. et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719–5730 (2002).
Udan, R. S., Kango-Singh, M., Nolo, R., Tao, C. & Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5, 914–920 (2003).
Harvey, K. F., Pfleger, C. M. & Hariharan, I. K. The Drosophila MST ortholog, Hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457–467 (2003).
Gaspar, P. & Tapon, N. Sensing the local environment: actin architecture and Hippo signalling. Curr. Opin. Cell Biol. 31C, 74–83 (2014).
Zheng, Y. & Pan, D. The Hippo signaling pathway in development and disease. Dev. Cell 50, 264–282 (2019).
Meng, Z., Moroishi, T. & Guan, K. L. Mechanisms of Hippo pathway regulation. Genes Dev. 30, 1–17 (2016).
Moya, I. M. & Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Harvey, K. F., Zhang, X. & Thomas, D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Staley, B. K. & Irvine, K. D. Hippo signaling in Drosophila: recent advances and insights. Dev. Dyn. 241, 3–15 (2012).
Enderle, L. & McNeill, H. Hippo gains weight: added insights and complexity to pathway control. Sci. Signal. 6, re7 (2013).
Huang, J., Wu, S., Barrera, J., Matthews, K. & Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122, 421–434 (2005).
Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Oka, T., Mazack, V. & Sudol, M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J. Biol. Chem. 283, 27534–27546 (2008).
Sebé-Pedrós, A., Zheng, Y., Ruiz-Trillo, I. & Pan, D. Premetazoan origin of the Hippo signaling pathway. Cell Rep. 1, 13–20 (2012).
Ikmi, A. et al. Molecular evolution of the Yap/Yorkie proto-oncogene and elucidation of its core transcriptional program. Mol. Biol. Evol. 31, 1375–1390 (2014).
Mikeladze-Dvali, T. et al. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell 122, 775–787 (2005).
Nishioka, N. et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 (2009).
Wang, Y. et al. Comprehensive molecular characterization of the Hippo signaling pathway in cancer. Cell Rep. 25, 1304–1317.e1305 (2018).
Kulkarni, A., Chang, M. T., Vissers, J. H. A., Dey, A. & Harvey, K. F. The Hippo pathway as a driver of select human cancers. Trends Cancer 6, 781–796 (2020).
Zhou, D. et al. MST1 and MST2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the YAP1 oncogene. Cancer Cell 16, 425–438 (2009).
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007).
Lee, J. H. et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 27, 1231–1242 (2008).
Kobayashi, S., Cox, A. G., Harvey, K. F. & Hogan, B. M. Vasculature is getting Hip(po): Hippo signaling in vascular development and disease. Dev. Cell 58, 2627–2640 (2023).
Heallen, T. et al. Hippo pathway inhibits WNT signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011).
Watt, K. I. et al. The Hippo pathway effector YAP is a critical regulator of skeletal muscle fibre size. Nat. Commun. 6, 6048 (2015).
Cao, X., Pfaff, S. L. & Gage, F. H. YAP regulates neural progenitor cell number via the TEA ___domain transcription factor. Genes Dev. 22, 3320–3334 (2008).
Jukam, D. et al. Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science 342, 1238016 (2013).
Pojer, J. M., Manning, S. A., Kroeger, B., Kondo, S. & Harvey, K. F. The Hippo pathway uses different machinery to control cell fate and organ size. iScience 24, 102830 (2021).
Pojer, J. M., Saiful Hilmi, A. J., Kondo, S. & Harvey, K. F. Crumbs and the apical spectrin cytoskeleton regulate R8 cell fate in the Drosophila eye. PLoS Genet. 17, e1009146 (2021).
Jukam, D. & Desplan, C. Binary regulation of Hippo pathway by Merlin/NF2, Kibra, Lgl, and Melted specifies and maintains postmitotic neuronal fate. Dev. Cell 21, 874–887 (2011).
Kowalczyk, W. et al. Hippo signaling instructs ectopic but not normal organ growth. Science 378, eabg3679 (2022).
Halder, G., Dupont, S. & Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13, 591–600 (2012).
Sun, S. & Irvine, K. D. Cellular organization and cytoskeletal regulation of the Hippo signaling network. Trends Cell Biol. 26, 694–704 (2016).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Wada, K., Itoga, K., Okano, T., Yonemura, S. & Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 (2011).
Rauskolb, C., Sun, S., Sun, G., Pan, Y. & Irvine, K. D. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell 158, 143–156 (2014).
Oh, H. et al. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep. 3, 309–318 (2013).
Oh, H. et al. Yorkie promotes transcription by recruiting a histone methyltransferase complex. Cell Rep. 8, 449–459 (2014).
Qing, Y. et al. The Hippo effector Yorkie activates transcription by interacting with a histone methyltransferase complex through Ncoa6. eLife 3, e02564 (2014).
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 (2015).
Galli, G. G. et al. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Mol. Cell 60, 328–337 (2015).
Goulev, Y. et al. Scalloped interacts with Yorkie, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 18, 435–441 (2008).
Ota, M. & Sasaki, H. Mammalian tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development 135, 4059–4069 (2008).
Wu, S., Liu, Y., Zheng, Y., Dong, J. & Pan, D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 14, 388–398 (2008).
Zhang, L. et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 14, 377–387 (2008).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008).
Koontz, L. M. et al. The Hippo effector Yorkie controls normal tissue growth by antagonizing Scalloped-mediated default repression. Dev. Cell 25, 388–401 (2013).
Guo, T. et al. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. 23, 1201–1214 (2013).
Vissers, J. H. A. et al. The Scalloped and Nerfin-1 transcription factors cooperate to maintain neuronal cell fate. Cell Rep. 25, 1561–1576.e1567 (2018).
Guo, P. et al. Nerfin-1 represses transcriptional output of Hippo signaling in cell competition. eLife 8, e38843 (2019).
Vissers, J. H. A., Dent, L. G., House, C. M., Kondo, S. & Harvey, K. F. Pits and CtBP control tissue growth in Drosophila melanogaster with the Hippo pathway transcription repressor Tgi. Genetics 215, 117–128 (2020).
Zhang, W. et al. The TEA ___domain family transcription factor TEAD4 represses murine adipogenesis by recruiting the cofactors VGLL4 and CtBP2 into a transcriptional complex. J. Biol. Chem. 293, 17119–17134 (2018).
Manning, S. A. et al. The Drosophila Hippo pathway transcription factor Scalloped and its co-factors alter each other’s chromatin binding dynamics and transcription in vivo. Dev. Cell 59, 1640–1654.e1645 (2024).
Kroeger, B. et al. Hippo signalling regulates the nuclear behaviour and DNA dwell times of YAP and TEAD to control transcription. Preprint at https://doi.org/10.1101/2025.03.11.642705 (2025).
Asrani, K. et al. Reciprocal YAP1 loss and INSM1 expression in neuroendocrine prostate cancer. J. Pathol. 255, 425–437 (2021).
Halder, G. et al. The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 12, 3900–3909 (1998).
Paumard-Rigal, S., Zider, A., Vaudin, P. & Silber, J. Specific interactions between Vestigial and Scalloped are required to promote wing tissue proliferation in Drosophila melanogaster. Dev. Genes Evol. 208, 440–446 (1998).
Zider, A., Paumard-Rigal, S., Frouin, I. & Silber, J. The vestigial gene of Drosophila melanogaster is involved in the formation of the peripheral nervous system: genetic interactions with the scute gene. J. Neurogenet. 12, 87–99 (1998).
Sonnemann, H. M., Pazdrak, B., Antunes, D. A., Roszik, J. & Lizee, G. Vestigial-like 1 (VGLL1): an ancient co-transcriptional activator linking wing, placenta, and tumor development. Biochim. Biophys. Acta Rev. Cancer 1878, 188892 (2023).
Simmonds, A. J. et al. Molecular interactions between Vestigial and Scalloped promote wing formation in Drosophila. Genes Dev. 12, 3815–3820 (1998).
Lai, Z. C. et al. Control of cell proliferation and apoptosis by Mob as tumor suppressor, Mats. Cell 120, 675–685 (2005).
Meng, Z. et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 6, 8357 (2015).
Zheng, Y. et al. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev. Cell 34, 642–655 (2015).
Boggiano, J. C., Vanderzalm, P. J. & Fehon, R. G. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev. Cell 21, 888–895 (2011).
Poon, C. L., Lin, J. I., Zhang, X. & Harvey, K. F. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev. Cell 21, 896–906 (2011).
Ribeiro, P. S. et al. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 39, 521–534 (2010).
Grusche, F. A., Richardson, H. E. & Harvey, K. F. Upstream regulation of the Hippo size control pathway. Curr. Biol. 20, R574–R582 (2010).
Su, T., Ludwig, M. Z., Xu, J. & Fehon, R. G. Kibra and Merlin activate the Hippo pathway spatially distinct from and independent of Expanded. Dev. Cell 40, 478–490.e473 (2017).
Sun, S., Reddy, B. V. & Irvine, K. D. Localization of Hippo signalling complexes and Warts activation in vivo. Nat. Commun. 6, 8402 (2015).
Angus, L. et al. Willin/FRMD6 expression activates the Hippo signaling pathway kinases in mammals and antagonizes oncogenic YAP. Oncogene 31, 238–250 (2012).
Guo, P. et al. PI4P-mediated solid-like Merlin condensates orchestrate Hippo pathway regulation. Science 385, eadf4478 (2024).
Li, F. L. et al. Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nat. Chem. Biol. 18, 1076–1086 (2022).
Grzeschik, N. A., Parsons, L. M., Allott, M. L., Harvey, K. F. & Richardson, H. E. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 (2010).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Robinson, B. S., Huang, J., Hong, Y. & Moberg, K. H. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-___domain protein expanded. Curr. Biol. 20, 582–590 (2010).
Ling, C. et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl Acad. Sci. USA 107, 10532–10537 (2010).
Chen, C. L. et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl Acad. Sci. USA 107, 15810–15815 (2010).
Varelas, X. et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev. Cell 19, 831–844 (2010).
Bossuyt, W. et al. An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene 33, 1218–1228 (2014).
Wang, C. et al. Integrated screens uncover a cell surface tumor suppressor gene KIRREL involved in Hippo pathway. Proc. Natl Acad. Sci. USA 119, e2121779119 (2022).
Gu, Y. et al. Transmembrane protein KIRREL1 regulates Hippo signaling via a feedback loop and represents a therapeutic target in YAP/TAZ-active cancers. Cell Rep. 40, 111296 (2022).
Deng, H. et al. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. eLife 4, e06567 (2015).
Fletcher, G. C. et al. The spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 34, 940–954 (2015).
Wong, K. K. et al. β-Spectrin regulates the hippo signaling pathway and modulates the basal actin network. J. Biol. Chem. 290, 6397–6407 (2015).
Ibar, C., Chinthalapudi, K., Heissler, S. M. & Irvine, K. D. Competition between myosin II and beta(H)-spectrin regulates cytoskeletal tension. eLife 12, RP84918 (2023).
Tyler, D. M. & Baker, N. E. Expanded and Fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev. Biol. 305, 187–201 (2007).
Willecke, M. et al. The Fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 16, 2090–2100 (2006).
Silva, E., Tsatskis, Y., Gardano, L., Tapon, N. & McNeill, H. The tumor-suppressor gene Fat controls tissue growth upstream of expanded in the Hippo signaling pathway. Curr. Biol. 16, 2081–2089 (2006).
Cho, E. et al. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 38, 1142–1150 (2006).
Bennett, F. C. & Harvey, K. F. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr. Biol. 16, 2101–2110 (2006).
Gridnev, A. & Misra, J. R. Emerging mechanisms of growth and patterning regulation by Dachsous and Fat protocadherins. Front. Cell Dev. Biol. 10, 842593 (2022).
Martin, D. et al. Assembly and activation of the Hippo signalome by FAT1 tumor suppressor. Nat. Commun. 9, 2372 (2018).
Lu, W. T. et al. TRACERx analysis identifies a role for FAT1 in regulating chromosomal instability and whole-genome doubling via Hippo signalling. Nat. Cell Biol. 27, 154–168 (2025).
Das Thakur, M. et al. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr. Biol. 20, 657–662 (2010).
Kroeger, B. et al. Basal spot junctions of Drosophila epithelial tissues respond to morphogenetic forces and regulate Hippo signaling. Dev. Cell 59, 262–279.e266 (2024).
Ibar, C. et al. Tension-dependent regulation of mammalian Hippo signaling through LIMD1. J. Cell Sci. 131, jcs214700 (2018).
Huang, H. L. et al. Par-1 regulates tissue growth by influencing Hippo phosphorylation status and Hippo-Salvador association. PLoS Biol. 11, e1001620 (2013).
Tokamov, S. A. et al. Cortical tension promotes Kibra degradation via Par-1. Mol. Biol. Cell 35, ar2 (2024).
Heidary Arash, E., Shiban, A., Song, S. & Attisano, L. MARK4 inhibits Hippo signaling to promote proliferation and migration of breast cancer cells. EMBO Rep. 18, 420–436 (2017).
Kwan, J. et al. DLG5 connects cell polarity and Hippo signaling protein networks by linking PAR-1 with MST1/2. Genes Dev. 30, 2696–2709 (2016).
Klingbeil, O. et al. MARK2/MARK3 kinases are catalytic codependencies of YAP/TAZ in human cancer. Cancer Discov. 14, 2471–2488 (2024).
Feng, Y. & Irvine, K. D. Fat and Expanded act in parallel to regulate growth through Warts. Proc. Natl Acad. Sci. USA 104, 20362–20367 (2007).
Qi, S. et al. Two Hippo signaling modules orchestrate liver size and tumorigenesis. EMBO J. 42, e115749 (2023).
Yu, J. et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev. Cell 18, 288–299 (2010).
Genevet, A., Wehr, M. C., Brain, R., Thompson, B. J. & Tapon, N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell 18, 300–308 (2010).
Baumgartner, R., Poernbacher, I., Buser, N., Hafen, E. & Stocker, H. The WW ___domain protein Kibra acts upstream of Hippo in Drosophila. Dev. Cell 18, 309–316 (2010).
Dey, A., Varelas, X. & Guan, K. L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 19, 480–494 (2020).
Wang, J., Liu, S., Heallen, T. & Martin, J. F. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 15, 672–684 (2018).
Petrilli, A. M. & Fernández-Valle, C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 35, 537–548 (2016).
Fossdal, R. et al. A novel TEAD1 mutation is the causative allele in Sveinsson’s chorioretinal atrophy (helicoid peripapillary chorioretinal degeneration). Hum. Mol. Genet. 13, 975–981 (2004).
Sanchez-Vega, F. et al. Oncogenic signaling pathways in the Cancer Genome Atlas. Cell 173, 321–337.e310 (2018).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Murakami, H. et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 71, 873–883 (2011).
Sekido, Y. Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol. Int. 61, 331–344 (2011).
Bueno, R. et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 48, 407–416 (2016).
Markowitz, P. et al. Genomic characterization of malignant pleural mesothelioma and associated clinical outcomes. Cancer Treat. Res. Commun. 25, 100232 (2020).
Sekido, Y. & Sato, T. NF2 alteration in mesothelioma. Front. Toxicol. 5, 1161995 (2023).
Tanas, M. R. et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci. Transl. Med. 3, 98ra82 (2011).
Errani, C. et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50, 644–653 (2011).
Merritt, N. et al. TAZ-CAMTA1 and YAP-TFE3 alter the TAZ/YAP transcriptome by recruiting the ATAC histone acetyltransferase complex. eLife 10, e62857 (2021).
Seavey, C. N., Pobbati, A. V. & Rubin, B. P. Unraveling the biology of epithelioid hemangioendothelioma, a TAZ-CAMTA1 fusion driven sarcoma. Cancers 14, 2980 (2022).
Garcia, K., Gingras, A. C., Harvey, K. F. & Tanas, M. R. TAZ/YAP fusion proteins: mechanistic insights and therapeutic opportunities. Trends Cancer 8, 1033–1045 (2022).
Agaimy, A. et al. Recurrent VGLL3 fusions define a distinctive subset of spindle cell rhabdomyosarcoma with an indolent clinical course and striking predilection for the head and neck. Genes Chromosomes Cancer 61, 701–709 (2022).
Guo, S. et al. VGLL2 and TEAD1 fusion proteins identified in human sarcoma drive YAP/TAZ-independent tumorigenesis by engaging EP300. eLife 13, 98386 (2025).
Pearson, J. D. et al. Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity. Cancer Cell 39, 1115–1134.e1112 (2021).
Marine, J. C., Dawson, S. J. & Dawson, M. A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer 20, 743–756 (2020).
Nguyen, C. D. K. & Yi, C. YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer 5, 283–296 (2019).
Kapoor, A. et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 158, 185–197 (2014).
Shao, D. D. et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158, 171–184 (2014).
Lin, L. et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat. Genet. 47, 250–256 (2015).
Hagenbeek, T. J. et al. An allosteric pan-TEAD inhibitor blocks oncogenic YAP/TAZ signaling and overcomes KRAS G12C inhibitor resistance. Nat. Cancer 4, 812–828 (2023).
Mukhopadhyay, S. et al. Genome-wide CRISPR screens identify multiple synthetic lethal targets that enhance KRASG12C inhibitor efficacy. Cancer Res. 83, 4095–4111 (2023).
Tape, C. J. Plastic persisters: revival stem cells in colorectal cancer. Trends Cancer 10, 185–195 (2024).
Han, T. et al. Lineage reversion drives WNT independence in intestinal cancer. Cancer Discov. 10, 1590–1609 (2020).
Qin, X. et al. An oncogenic phenoscape of colonic stem cell polarization. Cell 186, 5554–5568.e5518 (2023).
Rebekah, M. et al. Patient-derived colorectal cancer organoids upregulate revival stem cell marker genes following chemotherapeutic treatment. J. Clin. Med. 9, 128 (2020).
Ayyaz, A. et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125 (2019).
Fan, F. et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci. Transl. Med. 8, 352ra108 (2016).
Zhang, P. et al. Exploration of MST1-mediated secondary brain injury induced by intracerebral hemorrhage in Rats via Hippo signaling pathway. Transl. Stroke Res. 10, 729–743 (2019).
Wu, Y. et al. Discovery of IHMT-MST1-58 as a novel, potent, and selective MST1 inhibitor for the treatment of type 1/2 diabetes. J. Med. Chem. 65, 11818–11839 (2022).
Kastan, N. et al. Small-molecule inhibition of Lats kinases may promote Yap-dependent proliferation in postmitotic mammalian tissues. Nat. Commun. 12, 3100 (2021).
Kastan, N. R. et al. Development of an improved inhibitor of Lats kinases to promote regeneration of mammalian organs. Proc. Natl Acad. Sci. 119, e2206113119 (2022).
Aihara, A. et al. Small molecule LATS kinase inhibitors block the Hippo signaling pathway and promote cell growth under 3D culture conditions. J. Biol. Chem. 298, 101779 (2022).
Issabayeva, G. et al. Discovery of selective LATS inhibitors via scaffold hopping: enhancing drug-likeness and kinase selectivity for potential applications in regenerative medicine. RSC Med. Chem. 15, 4080–4089 (2024).
Namoto, K. et al. NIBR-LTSi is a selective LATS kinase inhibitor activating YAP signaling and expanding tissue stem cells in vitro and in vivo. Cell Stem Cell 31, 554–569.e517 (2024).
Burgess, C. L. et al. Generation of human alveolar epithelial type I cells from pluripotent stem cells. Cell Stem Cell 31, 657–675.e658 (2024).
Dost, A. F. M. et al. A human organoid model of alveolar regeneration reveals distinct epithelial responses to interferon-gamma. Preprint at https://doi.org/10.1101/2025.01.30.635624 (2025).
Liu, S. et al. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci. Transl. Med. 13, eabd6892 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/search?term=NCT06831825 (2025).
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012).
Kim, N. G. & Gumbiner, B. M. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J. Cell Biol. 210, 503–515 (2015).
Woodard, G. A., Yang, Y. L., You, L. & Jablons, D. M. Drug development against the Hippo pathway in mesothelioma. Transl. Lung Cancer Res. 6, 335–342 (2017).
Dhanaraman, T. et al. RASSF effectors couple diverse RAS subfamily GTPases to the Hippo pathway. Sci. Signal. 13, eabb4778 (2020).
Gill, M. K. et al. A feed forward loop enforces YAP/TAZ signaling during tumorigenesis. Nat. Commun. 9, 3510 (2018).
Yuan, W. C. et al. NUAK2 is a critical YAP target in liver cancer. Nat. Commun. 9, 4834 (2018).
Port, J. et al. Colorectal tumors require NUAK1 for protection from oxidative stress. Cancer Discov. 8, 632–647 (2018).
Skalka, G. L., Whyte, D., Lubawska, D. & Murphy, D. J. NUAK: never underestimate a kinase. Essays Biochem. 68, 295–307 (2024).
Graham, K. et al. Discovery of YAP1/TAZ pathway inhibitors through phenotypic screening with potent anti-tumor activity via blockade of Rho-GTPase signaling. Cell Chem. Biol. 31, 1247–1263.e1216 (2024).
Macleod, A. R. The discovery and characterization of ION-537: a next generation antisense oligonucleotide inhibitor of YAP1 in preclinical cancer models. Cancer Res. 81 (Suppl. 13), Abstr. ND11 (2021).
Zhou, C. et al. Exploring degradation of intrinsically disordered protein Yes-associated protein induced by proteolysis targeting chimeras. J. Med. Chem. 67, 15168–15198 (2024).
Barbosa, I. A. M. et al. Cancer lineage-specific regulation of YAP responsive elements revealed through large-scale functional epigenomic screens. Nat. Commun. 14, 3907 (2023).
Pobbati, A. V. et al. Targeting the central pocket in human transcription factor TEAD as a potential cancer therapeutic strategy. Structure 23, 2076–2086 (2015).
Chan, P. et al. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat. Chem. Biol. 12, 282–289 (2016).
Noland, C. L. et al. Palmitoylation of TEAD transcription factors is required for their stability and function in Hippo pathway signaling. Structure 24, 179–186 (2016).
Baroja, I., Kyriakidis, N. C., Halder, G. & Moya, I. M. Expected and unexpected effects after systemic inhibition of Hippo transcriptional output in cancer. Nat. Commun. 15, 2700 (2024).
Brennan, D., Liang, Y., Mlynarski, S. & Zhu, B.-Y. in 2024 Medicinal Chemistry Reviews (vol. 59) Ch. 9, 175-201 (ACS, 2024).
Pobbati, A. V., Kumar, R., Rubin, B. P. & Hong, W. Therapeutic targeting of TEAD transcription factors in cancer. Trends Biochem. Sci. 48, 450–462 (2023).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e516 (2017).
Tang, T. T. et al. Small molecule inhibitors of TEAD auto-palmitoylation selectively inhibit proliferation and tumor growth of NF2-deficient mesothelioma. Mol. Cancer Ther. 20, 986–998 (2021).
Lu, W. et al. Discovery and biological evaluation of vinylsulfonamide derivatives as highly potent, covalent TEAD autopalmitoylation inhibitors. Eur. J. Med. Chem. 184, 111767 (2019).
Li, Q. et al. Lats1/2 sustain intestinal stem cells and Wnt activation through TEAD-dependent and independent transcription. Cell Stem Cell 26, 675–692.e678 (2020).
Kaneda, A. et al. The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am. J. Cancer Res. 10, 4399–4415 (2020).
Heinrich, T. et al. Optimization of TEAD P-site binding fragment hit into in vivo active lead MSC-4106. J. Med. Chem. 65, 9206–9229 (2022).
Lu, W. et al. Structure-based design of Y-shaped covalent TEAD inhibitors. J. Med. Chem. 66, 4617–4632 (2023).
Hillen, H. et al. A novel irreversible TEAD inhibitor, SWTX-143, blocks Hippo pathway transcriptional output and causes tumor regression in preclinical mesothelioma models. Mol. Cancer Ther. 23, 3–13 (2024).
Young, N. et al. Abstract 1646: IK-930, a paralog-selective TEAD inhibitor for treating YAP/TAZ-TEAD dependent cancers. Cancer Res. 83 (Suppl. 7), 1646 (2023).
Gordon, J. A. et al. Abstract 6589: Discovery of potent and selective pan-TEAD autopalmitoylation inhibitors for the treatment of Hippo-pathway altered cancers. Cancer Res. 84 (Suppl. 6), 6589 (2024).
Guo, S. et al. Abstract 4976: Preclinical characterization of BGI-9004, a covalent TEAD inhibitor with exceptional anti-cancer activity and combination potential. Cancer Res. 83 (Suppl. 7), 4976 (2023).
Han, X. et al. Abstract 7575: BPI-460372, a covalent, irreversible TEAD inhibitor in phase I clinical development. Cancer Res. 84 (Suppl. 6), 7575 (2024).
Chen, P.-Y. et al. Abstract 7264: OPN-9840, a non-covalent potent pan-TEAD inhibitor, exhibits single agent efficacy in preclinical malignant mesothelioma models. Cancer Res. 84 (Suppl. 6), 7264 (2024).
Lu, J. et al. Abstract 7265: ETS-005, a highly selective TEAD4 palmitoylation inhibitor with potent anti-tumor activity and brain penetrating capability. Cancer Res. 84 (Suppl. 6), 7265 (2024).
Muller, F., Kunnimalaiyaan, S., Mangrolia, P. & Olson, J. Abstract 5913: TEAD1/4 inhibitors exhibit deeper biological impact and broader activity compared to TEAD1-only inhibitors in both monotherapy and combination without additional kidney toxicity. Cancer Res. 84 (Suppl. 6), 5913 (2024).
Kim, J. et al. Pan-transcriptional enhanced associated ___domain palmitoylation pocket covalent inhibitor. J. Med. Chem. 67, 18957–18968 (2024).
Moure, C. J. et al. Activation of hepatocyte growth factor/MET signaling as a mechanism of acquired resistance to a novel YAP1/TEAD small molecule inhibitor. Mol. Cancer Ther. 23, 1095–1108 (2024).
Heinrich, T. et al. MoA studies of the TEAD P-site binding ligand MSC-4106 and its optimization to TEAD1-selective amide M3686. J. Med. Chem. 68, 6149–6164 (2025).
Kurppa, K. J. et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell 37, 104–122.e112 (2020).
Bum-Erdene, K. et al. Small-molecule covalent modification of conserved cysteine leads to allosteric inhibition of the TEAD⋅Yap protein-protein interaction. Cell Chem. Biol. 26, 378–389.e313 (2019).
Yap, T. A. et al. Abstract CT006: First-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of Yes-associated protein (YAP)/transcriptional enhancer activator ___domain (TEAD), in patients (pts) with advanced solid tumors enriched for malignant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations. Cancer Res. 83 (Suppl. 8), CT006 (2023).
Yap, T. A. et al. First-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of Yes-Associated Protein (YAP)/Transcriptional EnhancerActivator Domain (TEAD), in patients with advanced solid tumors enriched for malignant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations. AACR Annual Meeting 2023 https://vivacetherapeutics.com/wp-content/uploads/Vivace-Therapeutics-2023-AACR-Presentation.pdf (2023).
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012).
Hau, J. C. et al. The TEAD4-YAP/TAZ protein-protein interaction: expected similarities and unexpected differences. Chembiochem 14, 1218–1225 (2013).
Zhang, Z. et al. Structure-based design and synthesis of potent cyclic peptides inhibiting the YAP-TEAD protein-protein interaction. ACS Med. Chem. Lett. 5, 993–998 (2014).
Mesrouze, Y. et al. Dissection of the interaction between the intrinsically disordered YAP protein and the transcription factor TEAD. eLife 6, e25068 (2017).
Furet, P. et al. Structure-based design of potent linear peptide inhibitors of the YAP-TEAD protein-protein interaction derived from the YAP omega-loop sequence. Bioorg. Med. Chem. Lett. 29, 2316–2319 (2019).
Furet, P. et al. The first class of small molecules potently disrupting the YAP-TEAD interaction by direct competition. ChemMedChem 17, e202200303 (2022).
Mesrouze, Y. et al. Biochemical and structural characterization of a peptidic inhibitor of the YAP:TEAD interaction that binds to the α-Helix pocket on TEAD. ACS Chem. Biol. 18, 643–651 (2023).
Sellner, H. et al. Optimization of a class of dihydrobenzofurane analogs toward orally efficacious YAP-TEAD protein-protein interaction inhibitors. ChemMedChem 18, e202300051 (2023).
Chapeau, E. A. et al. Direct and selective pharmacological disruption of the YAP–TEAD interface by IAG933 inhibits Hippo-dependent and RAS–MAPK-altered cancers. Nat. Cancer 5, 1102–1120 (2024).
Lu, J. et al. Abstract 4585: discovery of ETS-006, a highly potent YAP/TEADs PPI inhibitor with broad anti-tumor activity as a single agent. Cancer Res. 84, 4585(2024).
Karatas, H. et al. Discovery of covalent inhibitors targeting the transcriptional enhanced associate ___domain central pocket. J. Med. Chem. 63, 11972–11989 (2020).
Sawant, R. et al. Abstract LB029: Degraders of TEAD transcription factors based on interface 3 binders. Cancer Res. 84 (Suppl. 7), LB029 (2024).
Lu, Y. et al. Selective degradation of TEADs by a PROTAC molecule exhibited robust anticancer efficacy in vitro and in vivo. J. Med. Chem. 68, 5616–5640 (2025).
Chen, H. et al. Targeted degradation of specific TEAD paralogs by small molecule degraders. Heliyon 10, e37829 (2024).
Li, H. et al. Design, synthesis, and bioevaluation of transcriptional enhanced assocciated ___domain (TEAD) PROTAC degraders. ACS Med. Chem. Lett. 15, 631–639 (2024).
Deng, X. & Fang, L. VGLL4 is a transcriptional cofactor acting as a novel tumor suppressor via interacting with TEADs. Am. J. Cancer Res. 8, 932–943 (2018).
Cai, J. et al. YAP-VGLL4 antagonism defines the major physiological function of the Hippo signaling effector YAP. Genes Dev. 36, 1119–1128 (2022).
Zhao, B., Pobbati, A. V., Rubin, B. P. & Stauffer, S. Leveraging hot spots of TEAD-coregulator interactions in the design of direct small molecule protein-protein interaction disruptors targeting Hippo pathway signaling. Pharmaceuticals 16, 583 (2023).
Jiao, S. et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25, 166–180 (2014).
Kulkarni, A. et al. Identification of resistance mechanisms to small-molecule inhibition of TEAD-regulated transcription. EMBO Rep. 25, 3944–3969 (2024).
Guarnaccia, A. D. et al. TEAD-targeting small molecules induce a cofactor switch to regulate the Hippo pathway. Preprint at https://doi.org/10.1101/2024.11.15.623512 (2024).
Seavey, C. N. et al. Loss of CDKN2A cooperates with WWTR1(TAZ)-CAMTA1 gene fusion to promote tumor progression in epithelioid hemangioendothelioma. Clin. Cancer Res. 29, 2480–2493 (2023).
Zhang, F. et al. Recurrent RhoGAP gene fusion CLDN18-ARHGAP26 promotes RHOA activation and focal adhesion kinase and YAP-TEAD signalling in diffuse gastric cancer. Gut 73, 1280–1291 (2024).
Saito, Y. et al. A therapeutically targetable TAZ-TEAD2 pathway drives the growth of hepatocellular carcinoma via ANLN and KIF23. Gastroenterology 164, 1279–1292 (2023).
Holden, J. K. et al. Small molecule dysregulation of TEAD lipidation induces a dominant-negative inhibition of Hippo pathway signaling. Cell Rep. 31, 107809 (2020).
Sato, K. et al. Targeting YAP/TAZ-TEAD signaling as a therapeutic approach in head and neck squamous cell carcinoma. Cancer Lett. 612, 217467 (2025).
Murakami, S., White, S. M., McIntosh, A. T., Nguyen, C. D. K. & Yi, C. Spontaneously evolved progenitor niches escape Yap oncogene addiction in advanced pancreatic ductal adenocarcinomas. Nat. Commun. 14, 1443 (2023).
Barrette, A. M. et al. Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models. Neuro Oncol. 24, 694–707 (2022).
Laraba, L. et al. Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma. Brain 146, 1697–1713 (2023).
Press release: Ikena Oncology shares initial positive and differentiated dose escalation data from IK-930 phase I trial and reports third quarter 2023 financial results. Ikena Oncology https://ir.ikenaoncology.com/node/7936/pdf (2023).
Press Release: Ikena Oncology announes strategic update. Ikena Oncology https://ir.ikenaoncology.com/node/8131/pdf (2024).
Tang, T. T. & Post, L. Abstract 7282: Comparing TEAD palmitoylation inhibitors with differential TEAD selectivity in combination efficacy with targeted therapies and in renal safety. Cancer Res. 84 (Suppl. 6), 7282 (2024).
Hashimoto, M. & Sasaki, H. Epiblast formation by TEAD-YAP-dependent expression of pluripotency factors and competitive elimination of unspecified cells. Dev. Cell 50, 139–154.e135 (2019).
Nishioka, N. et al. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech. Dev. 125, 270–283 (2008).
Sawada, A. et al. Redundant roles of Tead1 and Tead2 in notochord development and the regulation of cell proliferation and survival. Mol. Cell Biol. 28, 3177–3189 (2008).
Chen, Z., Friedrich, G. A. & Soriano, P. Transcriptional enhancer factor 1 disruption by a retroviral gene trap leads to heart defects and embryonic lethality in mice. Genes Dev. 8, 2293–2301 (1994).
Kaneko, K. J., Kohn, M. J., Liu, C. & DePamphilis, M. L. Transcription factor TEAD2 is involved in neural tube closure. Genesis 45, 577–587 (2007).
Yagi, R. et al. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development 134, 3827–3836 (2007).
Kakiuchi-Kiyota, S., Schutten, M. M., Zhong, Y., Crawford, J. J. & Dey, A. Safety considerations in the development of Hippo pathway inhibitors in cancers. Front. Cell Dev. Biol. 7, 156 (2019).
Wong, J. S., Meliambro, K., Ray, J. & Campbell, K. N. Hippo signaling in the kidney: the good and the bad. Am. J. Physiol. Ren. Physiol. 311, F241–F248 (2016).
Schwartzman, M. et al. Podocyte-specific deletion of Yes-associated protein causes FSGS and progressive renal failure. J. Am. Soc. Nephrol. 27, 216–226 (2016).
Chen, J., Wang, X., He, Q. & Harris, R. C. TAZ is important for maintenance of the integrity of podocytes. Am. J. Physiol. Ren. Physiol. 322, F419–F428 (2022).
Pavenstädt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Chung, J. J. et al. Single-cell transcriptome profiling of the kidney glomerulus identifies key cell types and reactions to injury. J. Am. Soc. Nephrol. 31, 2341–2354 (2020).
Chen, J. et al. Inhibition of transcriptional coactivator YAP impairs the expression and function of transcription factor WT1 in diabetic podocyte injury. Kidney Int. 105, 1200–1211 (2024).
Rinschen, M. M. et al. YAP-mediated mechanotransduction determines the podocyte’s response to damage. Sci. Signal. 10, eaaf8165 (2017).
Haley, K. E. et al. YAP translocation precedes cytoskeletal rearrangement in podocyte stress response: a podometric investigation of diabetic nephropathy. Front. Physiol. 12, 625762 (2021).
Kaneda, A. et al. Abstract 3086: discovery of a first-in-class TEAD inhibitor which directly inhibits YAP/TAZ-TEAD protein-protein interaction and shows a potent anti-tumor effect in malignant pleural mesothelioma. Cancer Res. 79, 3086 (2019).
Paul, S., Sims, J., Pham, T. & Dey, A. Targeting the Hippo pathway in cancer: kidney toxicity as a class effect of TEAD inhibitors? Trends Cancer 11, 25–36 (2024).
Otsuki, H. et al. Reversible and monitorable nephrotoxicity in rats by the novel potent transcriptional enhanced associate ___domain (TEAD) inhibitor, K-975. J. Toxicol. Sci. 49, 175–191 (2024).
Sun, Y. et al. Pharmacological blockade of TEAD-YAP reveals its therapeutic limitation in cancer cells. Nat. Commun. 13, 6744 (2022).
Akao, K. et al. TEAD-independent cell growth of Hippo-inactive mesothelioma cells: unveiling resistance to TEAD inhibitor K-975 through MYC signaling activation. Mol. Cancer Ther. 24, 709–719 (2025).
Paul, S. et al. Cooperation between the Hippo and MAPK pathway activation drives acquired resistance to TEAD inhibition. Nat. Commun. 16, 1743 (2025).
Nutsch, K. et al. Augmented acyl-CoA biosynthesis promotes resistance to TEAD palmitoylation site inhibition. ACS Chem. Biol. 20, 967–975 (2025).
White, S. M. et al. YAP/TAZ inhibition induces metabolic and signaling rewiring resulting in targetable vulnerabilities in NF2-deficient tumor cells. Dev. Cell 49, 425–443.e429 (2019).
Coggins, G. E. et al. YAP1 mediates resistance to MEK1/2 inhibition in neuroblastomas with hyperactivated RAS signaling. Cancer Res. 79, 6204–6214 (2019).
Nilsson, M. B. et al. A YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance and increased expression of spindle assembly checkpoint components. Sci. Transl. Med. 12, eaaz4589 (2020).
Tsuji, T. et al. YAP1 mediates survival of ALK-rearranged lung cancer cells treated with alectinib via pro-apoptotic protein regulation. Nat. Commun. 11, 74 (2020).
Tang, T. T. & Post, L. Abstract B088: VT3989, the first-in-class and first-in-human TEAD auto-palmitoylation inhibitor, enhances the efficacy and durability of multiple targeted therapies of the MAPK and P13K/AKT/mTOR pathways. Mol. Cancer Ther. 22 (Suppl. 12), B088 (2023).
Edwards, A. C. et al. TEAD inhibition overcomes YAP1/TAZ-driven primary and acquired resistance to KRASG12C inhibitors. Cancer Res. 83, 4112–4129 (2023).
Ogimoto, T. et al. Combination therapy with EGFR tyrosine kinase inhibitors and TEAD inhibitor increases tumor suppression effects in EGFR mutation–positive lung cancer. Mol. Cancer Ther. 23, 564–576 (2024).
Haderk, F. et al. Focal adhesion kinase-YAP signaling axis drives drug-tolerant persister cells and residual disease in lung cancer. Nat. Commun. 15, 3741 (2024).
Wasko, U. N. et al. Tumour-selective activity of RAS-GTP inhibition in pancreatic cancer. Nature 629, 927–936 (2024).
Schirmer, A. et al. 234 (PB222): Rational combination of pan-TEAD inhibitor SW-682 and MEK inhibitor mirdametinib in head and neck squamous cell carcinomas leads to synergistic response. Eur. J. Cancer 211, 114752 (2024).
Chen, L. et al. 39 (PB027): TEAD inhibition by SW-682 potentiates activity of targeted therapies in NSCLC models. Eur. J. Cancer 211, 114567 (2024).
Hu, L. et al. Discovery of a new class of reversible TEA ___domain transcription factor inhibitors with a novel binding mode. eLife 11, e80210 (2022).
Liu, X. et al. In vitro and in vivo drug metabolism analysis of BPI-460372 - a covalent TEAD1/3/4 inhibitor. Curr. Drug Metab. 25, 754–768 (2025).
Marshall, C. J. Ras effectors. Curr. Opin. Cell Biol. 8, 197 (1996).
Adachi, Y. et al. Scribble mis-localization induces adaptive resistance to KRAS G12C inhibitors through feedback activation of MAPK signaling mediated by YAP-induced MRAS. Nat. Cancer 4, 829–843 (2023).
Pascual, J. et al. Hippo reprograms the transcriptional response to Ras signaling. Dev. Cell 42, 667–680.e664 (2017).
Mitchell, K. A. et al. The JNK and Hippo pathways control epithelial integrity and prevent tumor initiation by regulating an overlapping transcriptome. Curr. Biol. 34, 3966–3982.e3967 (2024).
Stein, C. et al. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 11, e1005465 (2015).
Pham, T. H. et al. Machine-learning and chemicogenomics approach defines and predicts cross-talk of Hippo and MAPK pathways. Cancer Discov. 11, 778–793 (2021).
Park, J. et al. YAP and AP-1 cooperate to initiate pancreatic cancer development from ductal cells in mice. Cancer Res. 80, 4768–4779 (2020).
Acknowledgements
K.F.H. holds a National Health and Medical Research Council of Australia Investigator Grant (APP1194467). The authors thank L. Post for comments on the manuscript and A. W. Konradi for drawing chemical structures.
Author information
Authors and Affiliations
Contributions
Both K.F.H. and T.T.T. analysed data, designed content, wrote and edited the manuscript, and reviewed and approved it before submission and publication.
Corresponding author
Ethics declarations
Competing interests
T.T.T. reports employment with Vivace Therapeutics and has equity interest in Vivace Therapeutics. K.F.H. declares no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Xaralabos Varelas, Satu Juhila and Georg Halder for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov
ISRCTN: https://www.isrctn.com
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harvey, K.F., Tang, T.T. Targeting the Hippo pathway in cancer. Nat Rev Drug Discov (2025). https://doi.org/10.1038/s41573-025-01234-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s41573-025-01234-0