Abstract
The identification of amylin as a glucoregulatory peptide hormone with roles in meal-ending satiation sparked a surge of experimental development, which culminated in the amylin mimetic drug pramlintide. Pramlintide was approved by the FDA in 2005 for the treatment of type 1 diabetes mellitus and insulin-requiring type 2 diabetes, and was also explored as a novel anti-obesity treatment. Despite this exciting potential, efforts to develop an amylin-based anti-obesity therapeutic stalled owing to challenges around dosage frequency, safety and formulation. Generally, anti-obesity therapies have displayed modest efficacy and mixed safety profiles, leaving a clear unmet clinical need that requires addressing. Advances in peptide chemistry have reinvigorated the amylin field by enabling the manufacture of effective new amylin-based molecules, resulting in therapeutics that are now on the cusp of approval. At present, there are growing concerns around GLP1 receptor agonist-based therapeutics, in particular their association with loss of lean body mass. Additionally, treatment of patients with overweight or obesity without associated comorbidities is increasingly common. The widespread pharmacotherapy of otherwise healthy populations with overweight or obesity with the goal of improving future health requires further regulatory and ethical consideration. This Review describes how amylin controls energy homeostasis and provides a current overview of amylin-based therapeutic development.
Key points
-
Amylin is a neuroendocrine peptide hormone, biosynthesized mainly in the pancreatic islet β-cells and co-secreted with insulin, that circulates in the blood and controls food intake, glucose homeostasis and energy metabolism.
-
There are at least three amylin receptors, AMY1, AMY2 and AMY3, each of which is composed of the calcitonin receptor bound to one of three corresponding receptor activity-modifying proteins.
-
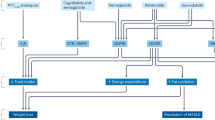
Amylin acts as a natural satiety signal that suppresses appetite, delays gastric emptying and reduces food intake by activating specific amylin receptors present in many regions of the brain.
-
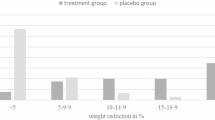
Treatment with amylin mimetics induces weight loss in humans and rodent models.
-
Amylin receptors are considered proven drug targets and amylin mimetics are emerging as novel treatments for overweight, obesity and diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ong, K. L. et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet 402, 203–234 (2023).
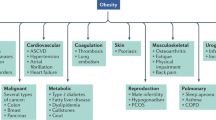
Lingvay, I., Cohen, R. V., le Roux, C. W. & Sumithran, P. Obesity in adults. Lancet 404, 972–987 (2024).
Linge, J., Birkenfeld, A. L. & Neeland, I. J. Muscle mass and glucagon-like peptide-1 receptor agonists: adaptive or maladaptive response to weight loss? Circulation 150, 1288–1298 (2024).
Abdullah bin Ahmed, I. A comprehensive review on weight gain following discontinuation of glucagon‐like peptide‐1 receptor agonists for obesity. J. Obes. 2024, 8056440 (2024).
Westermark, P. et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl Acad. Sci. USA 84, 3881–3885 (1987).
Fineman, M., Weyer, C., Maggs, D., Strobel, S. & Kolterman, O. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm. Metab. Res. 34, 504–508 (2002).
Smith, S. R. et al. Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care 31, 1816–1823 (2008).
Cooper, G. J. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocr. Rev. 15, 163–201 (1994).
Zhang, S. et al. The pathogenic mechanism of diabetes varies with the degree of overexpression and oligomerization of human amylin in the pancreatic islet β cells. FASEB J. 28, 5083–5096 (2014).
Hay, D. L., Chen, S., Lutz, T. A., Parkes, D. G. & Roth, J. D. Amylin: pharmacology, physiology, and clinical potential. Pharmacol. Rev. 67, 564–600 (2015).
Younk, L. M., Mikeladze, M. & Davis, S. N. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert. Opin. Pharmacother. 12, 1439–1451 (2011).
Aronne, L. et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J. Clin. Endocrinol. Metab. 92, 2977–2983 (2007).
Lau, D. C. et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet 398, 2160–2172 (2021).
Enebo, L. B. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet 397, 1736–1748 (2021).
Hay, D. L., Garelja, M. L., Poyner, D. R. & Walker, C. S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 175, 3–17 (2018).
Katafuchi, T., Yasue, H., Osaki, T. & Minamino, N. Calcitonin receptor-stimulating peptide: its evolutionary and functional relationship with calcitonin/calcitonin gene-related peptide based on gene structure. Peptides 30, 1753–1762 (2009).
Roberts, A. et al. Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc. Natl Acad. Sci. USA 86, 9662–9666 (1989).
Cooper, G. J. et al. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl Acad. Sci. USA 84, 8628–8632 (1987).
Westermark, P., Wernstedt, C., O’Brien, T. D., Hayden, D. W. & Johnson, K. H. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am. J. Pathol. 127, 414–417 (1987).
Ferrier, G. J. M. et al. Expression of the rat amylin (IAPP/DAP) gene. J. Mol. Endocrinol. 3, R1–R4 (1989).
Gebre-Medhin, S., Mulder, H., Zhang, Y., Sundler, F. & Betsholtz, C. Reduced nociceptive behavior in islet amyloid polypeptide (amylin) knockout mice. Brain Res. Mol. Brain Res. 63, 180–183 (1998).
Dobolyi, A. Central amylin expression and its induction in rat dams. J. Neurochem. 111, 1490–1500 (2009).
Rees, T. A. et al. Calcitonin receptor, calcitonin gene-related peptide and amylin distribution in C1/2 dorsal root ganglia. J. Headache Pain. 25, 36 (2024).
Hendrikse, E. R., Bower, R. L., Hay, D. L. & Walker, C. S. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 39, 403–419 (2019).
Hartter, E. et al. Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Diabetologia 34, 52–54 (1991).
Moore, C. X. & Cooper, G. J. Co-secretion of amylin and insulin from cultured islet β-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem. Biophys. Res. Commun. 179, 1–9 (1991).
Banks, W. A. & Kastin, A. J. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19, 883–889 (1998).
Banks, W. A., Kastin, A. J., Maness, L. M., Huang, W. & Jaspan, J. B. Permeability of the blood-brain barrier to amylin. Life Sci. 57, 1993–2001 (1995).
Leckstrom, A., Bjorklund, K., Permert, J., Larsson, R. & Westermark, P. Renal elimination of islet amyloid polypeptide. Biochem. Biophys. Res. Commun. 239, 265–268 (1997).
McLatchie, L. M. et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339 (1998).
Christopoulos, G. et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 56, 235–242 (1999).
Muff, R., Buhlmann, N., Fischer, J. A. & Born, W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology 140, 2924–2927 (1999).
Walker, C. S. et al. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann. Clin. Transl. Neurol. 2, 595–608 (2015).
Qi, T. et al. Receptor activity-modifying protein-dependent impairment of calcitonin receptor splice variant Δ(1–47)hCT(a) function. Br. J. Pharmacol. 168, 644–657 (2013).
Tilakaratne, N., Christopoulos, G., Zumpe, E. T., Foord, S. M. & Sexton, P. M. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J. Pharmacol. Exp. Ther. 294, 61–72 (2000).
Bailey, R. J. et al. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br. J. Pharmacol. 166, 151–167 (2012).
Garelja, M. L., Walker, C. S. & Hay, D. L. CGRP receptor antagonists for migraine. Are they also AMY1 receptor antagonists? Br. J. Pharmacol. 179, 454–459 (2022).
Liberini, C. G. et al. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur. J. Neurosci. 43, 653–661 (2016).
Le Foll, C. et al. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 64, 1621–1631 (2015).
Turek, V. F. et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 151, 143–152 (2010).
Hay, D. L. et al. Receptor activity-modifying proteins; multifunctional G protein-coupled receptor accessory proteins. Biochem. Soc. Trans. 44, 568–573 (2016).
Cegla, J. et al. RAMP2 influences glucagon receptor pharmacology via trafficking and signaling. Endocrinology 158, 2680–2693 (2017).
Kumar, K. K. et al. Negative allosteric modulation of the glucagon receptor by RAMP2. Cell 186, 1465–1477 (2023).
Shao, L. et al. Modulating effects of RAMPs on signaling profiles of the glucagon receptor family. Acta Pharm. Sin. B 12, 637–650 (2022).
Dackor, R., Fritz-Six, K., Smithies, O. & Caron, K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J. Biol. Chem. 282, 18094–18099 (2007).
Hosono, K. et al. Deletion of RAMP1 signaling enhances diet-induced obesity and fat absorption via intestinal lacteals in mice. In Vivo 38, 160–173 (2024).
Coester, B. et al. RAMP1 and RAMP3 differentially control amylin’s effects on food intake, glucose and energy balance in male and female mice. Neuroscience 447, 74–93 (2020).
Fernandes-Santos, C. et al. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology 154, 2481–2488 (2013).
Zhang, Z. et al. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes 60, 1063–1071 (2011).
Arrigoni, S. et al. A selective role for receptor activity-modifying proteins in subchronic action of the amylin selective receptor agonist NN1213 compared with salmon calcitonin on body weight and food intake in male mice. Eur. J. Neurosci. 54, 4863–4876 (2021).
Liu, T. et al. RAMP3 deficiency enhances postmenopausal obesity and metabolic disorders. Peptides 110, 10–18 (2018).
Westermark, P., Andersson, A. & Westermark, G. T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 91, 795–826 (2011).
Lutz, T. A. The role of amylin in the control of energy homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1475–R1484 (2010).
Paxinos, G. et al. In vitro autoradiographic localization of calcitonin and amylin binding sites in monkey brain. J. Chem. Neuroanat. 27, 217–236 (2004).
Sexton, P. M., Paxinos, G., Kenney, M. A., Wookey, P. J. & Beaumont, K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience 62, 553–567 (1994).
Beaumont, K., Kenney, M. A., Young, A. A. & Rink, T. J. High affinity amylin binding sites in rat brain. Mol. Pharmacol. 44, 493–497 (1993).
Becskei, C., Riediger, T., Zund, D., Wookey, P. & Lutz, T. A. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 1030, 221–233 (2004).
Oliver, K. R. et al. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur. J. Neurosci. 14, 618–628 (2001).
Skofitsch, G., Wimalawansa, S. J., Jacobowitz, D. M. & Gubisch, W. Comparative immunohistochemical distribution of amylin-like and calcitonin gene related peptide like immunoreactivity in the rat central nervous system. Can. J. Physiol. Pharmacol. 73, 945–956 (1995).
Hendrikse, E. R. et al. Calcitonin receptor antibody validation and expression in the rodent brain. Cephalalgia 42, 815–826 (2022).
Van Dyken, P. & Lacoste, B. Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Front. Neurosci. 12, 930 (2018).
Barth, S. W., Riediger, T., Lutz, T. A. & Rechkemmer, G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res. 997, 97–102 (2004).
Clarke, G. S., Page, A. J. & Eldeghaidy, S. The gut–brain axis in appetite, satiety, food intake, and eating behavior: insights from animal models and human studies. Pharmacol. Res. Perspect. 12, e70027 (2024).
Young, A., Gedulin, B., Vine, W., Percy, A. & Rink, T. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia 38, 642–648 (1995).
Gedulin, B. R. & Young, A. A. Hypoglycemia overrides amylin-mediated regulation of gastric emptying in rats. Diabetes 47, 93–97 (1998).
Mack, C. M. et al. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int. J. Obes. 34, 385–395 (2010).
Mollet, A., Gilg, S., Riediger, T. & Lutz, T. A. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol. Behav. 81, 149–155 (2004).
Chapman, I. et al. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia 48, 838–848 (2005).
Chapman, I. et al. Low‐dose pramlintide reduced food intake and meal duration in healthy, normal‐weight subjects. Obesity 15, 1179–1186 (2007).
Smith, S. R. et al. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am. J. Physiol. Endocrinol. Metab. 293, E620–E627 (2007).
Lutz, T., Del Prete, E. & Scharrer, E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol. Behav. 55, 891–895 (1994).
Morley, J. E., Suarez, M. D., Mattamal, M. & Flood, J. F. Amylin and food intake in mice: effects on motivation to eat and mechanism of action. Pharmacol. Biochem. Behav. 56, 123–129 (1997).
Riediger, T., Rauch, M. & Schmid, H. A. Actions of amylin on subfornical organ neurons and on drinking behavior in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 276, R514–R521 (1999).
Baldo, B. A. & Kelley, A. E. Amylin infusion into rat nucleus accumbens potently depresses motor activity and ingestive behavior. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1232–R1242 (2001).
Mietlicki-Baase, E. G. et al. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology 38, 1685–1697 (2013).
Mietlicki-Baase, E. G., Olivos, D. R., Jeffrey, B. A. & Hayes, M. R. Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. Am. J. Physiol. Endocrinol. Metab. 308, E1116–E1122 (2015).
Szabó, É. R., Cservenák, M. & Dobolyi, A. Amylin is a novel neuropeptide with potential maternal functions in the rat. FASEB J. 26, 272–281 (2012).
Trevaskis, J. L. et al. Interaction of leptin and amylin in the long‐term maintenance of weight loss in diet‐induced obese rats. Obesity 18, 21–26 (2010).
Aronoff, S. L., Berkowitz, K., Shreiner, B. & Want, L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr. 17, 183–190 (2004).
Honegger, M., Lutz, T. A. & Boyle, C. N. Hypoglycemia attenuates acute amylin-induced reduction of food intake in male rats. Physiol. Behav. 237, 113435 (2021).
Leighton, B. & Cooper, G. J. The role of amylin in the insulin resistance of non-insulin-dependent diabetes mellitus. Trends Biochem. Sci. 15, 295–299 (1990).
Gedulin, B. R., Jodka, C. M., Herrmann, K. & Young, A. A. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul. Pept. 137, 121–127 (2006).
Silvestre, R. A., Peiró, E., Dégano, P., Miralles, P. & Marco, J. Inhibitory effect of rat amylin on the insulin responses to glucose and arginine in the perfused rat pancreas. Regul. Pept. 31, 23–31 (1990).
Silvestre, R. et al. Selective amylin inhibition of the glucagon response to arginine is extrinsic to the pancreas. Am. J. Physiol. Endocrinol. Metab. 280, E443–E449 (2001).
Molina, J. M., Cooper, G. J., Leighton, B. & Olefsky, J. M. Induction of insulin resistance in vivo by amylin and calcitonin gene-related peptide. Diabetes 39, 260–265 (1990).
Nishimura, S. et al. Lack of effect of islet amyloid polypeptide on hepatic glucose output in the in situ-perfused rat liver. Metabolism 41, 431–434 (1992).
Sanke, T. et al. Plasma islet amyloid polypeptide (Amylin) levels and their responses to oral glucose in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 34, 129–132 (1991).
Young, A. A., Wang, M.-W. & Cooper, G. J. Amylin injection causes elevated plasma lactate and glucose in the rat. FEBS Lett. 291, 101–104 (1991).
Wang, M.-W., Carlo, P., Rink, T. J. & Young, A. A. Amylin is more potent and more effective than glucagon in raising plasma glucose concentration in fasted, anesthetized rats. Biochem. Biophys. Res. Commun. 181, 1288–1293 (1991).
Young, D., Deems, R., Deacon, R., McIntosh, R. & Foley, J. Effects of amylin on glucose metabolism and glycogenolysis in vivo and in vitro. Am. J. Physiol. Endocrinol. Metab. 259, E457 (1990).
Cori, C. F. Mammalian carbohydrate metabolism. Physiol. Rev. 11, 143–275 (1931).
Ellingwood, S. S. & Cheng, A. Biochemical and clinical aspects of glycogen storage diseases. J. Endocrinol. 238, R131–R141 (2018).
Leighton, B. & Cooper, G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature 335, 632–635 (1988).
Kreutter, D. K. et al. Amylin and CGRP induce insulin resistance via a receptor distinct from cAMP-coupled CGRP receptor. Am. J. Physiol. Endocrinol. Metab. 264, E606–E613 (1993).
Leighton, B. & Foot, E. The effects of amylin on carbohydrate metabolism in skeletal muscle in vitro and in vivo. Biochem. J. 269, 19–23 (1990).
Hothesall, J. S., Muirhead, R. P. & Wimalawansa, S. The effect of amylin and calcitonin gene-related peptide on insulin-stimulated glucose transport in the diaphragm. Biochem. Biophys. Res. Commun. 169, 451–454 (1990).
Danaher, R. N. et al. Evidence that α-calcitonin gene-related peptide is a neurohormone that controls systemic lipid availability and utilization. Endocrinology 149, 154–160 (2008).
Ye, J.-M. et al. Evidence that amylin stimulates lipolysis in vivo: a possible mediator of induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 280, E562–E569 (2001).
Chantry, A., Leighton, B. & Day, A. J. Cross-reactivity of amylin with calcitonin-gene-related peptide binding sites in rat liver and skeletal muscle membranes. Biochem. J. 277, 139–143 (1991).
Zhu, G., Dudley, D. T. & Saltiel, A. R. Amylin increases cyclic AMP formation in L6 myocytes through calcitonin gene-related peptide receptors. Biochem. Biophys. Res. Commun. 177, 771–776 (1991).
Gudzune, K. A. & Kushner, R. F. Medications for obesity: a review. JAMA 332, 571–584 (2024).
Williams, D. M., Nawaz, A. & Evans, M. Drug therapy in obesity: a review of current and emerging treatments. Diabetes Ther. 11, 1199–1216 (2020).
Cummings, D. E., Overduin, J. & Foster-Schubert, K. E. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J. Clin. Endocrinol. Metab. 89, 2608–2615 (2004).
Holst, J. J. GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management. Nat. Metab. 6, 1866–1885 (2024).
Drucker, D. J. Discovery of GLP-1-based drugs for the treatment of obesity. N. Engl. J. Med. 392, 612–615 (2025).
McGowan, B. M. et al. Efficacy and safety of once-weekly semaglutide 2·4 mg versus placebo in people with obesity and prediabetes (STEP 10): a randomised, double-blind, placebo-controlled, multicentre phase 3 trial. Lancet Diabetes Endocrinol. 12, 631–642 (2024).
Guglielmi, G. The weight-loss drugs being tested in 2025: will they beat Ozempic? Nature 638, 591–592 (2025).
Sargeant, J. A. et al. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol. Metab. 34, 247–262 (2019).
Ryan, G. J., Jobe, L. J. & Martin, R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin. Ther. 27, 1500–1512 (2005).
Roth, J. D., Hughes, H., Kendall, E., Baron, A. D. & Anderson, C. M. Antiobesity effects of the β-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 147, 5855–5864 (2006).
Gydesen, S. et al. Optimization of tolerability and efficacy of the novel dual amylin and calcitonin receptor agonist KBP-089 through dose escalation and combination with a GLP-1 analog. Am. J. Physiol. Endocrinol. Metab. 313, E598–E607 (2017).
Srinivasan, A., Wong, F. K. & Karponis, D. Calcitonin: a useful old friend. J. Musculoskelet. Neuronal Interact. 20, 600–609 (2020).
Wüster, C. et al. Superior local tolerability of human versus salmon calcitonin preparations in young healthy volunteers. Eur. J. Clin. Pharmacol. 41, 211–215 (1991).
Reginster, J. Y., Gaspar, S., Deroisy, R., Zegels, B. & Franchimont, P. Prevention of osteoporosis with nasal salmon calcitonin: effect of anti-salmon calcitonin antibody formation. Osteoporos. Int. 3, 261–264 (1993).
Zakariassen, H. L., John, L. M. & Lutz, T. A. Central control of energy balance by amylin and calcitonin receptor agonists and their potential for treatment of metabolic diseases. Basic. Clin. Pharmacol. Toxicol. 127, 163–177 (2020).
Jonderko, G., Gołąb, T. & Jonderko, K. Effect of calcitonin on gastric emptying. Digestion 40, 191–196 (1988).
Jonderko, K., Jonderko, G. & Golab, T. Effect of calcitonin on gastric emptying and on serum insulin and gastrin concentrations after ingestion of a mixed solid–liquid meal in humans. J. Clin. Gastroenterol. 12, 22–28 (1990).
Perlow, M. J., Freed, W. J., Carman, J. S. & Wyatt, R. J. Calcitonin reduces feeding in man, monkey and rat. Pharmacol. Biochem. Behav. 12, 609–612 (1980).
Gingell, J. J., Burns, E. R. & Hay, D. L. Activity of pramlintide, rat and human amylin but not Aβ1-42 at human amylin receptors. Endocrinology 155, 21–26 (2014).
Center for Drug Evaluation and Research. Symlin® (pramlintide acetate) injection: approved labeling (application no. 021332). FDA www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21-332_Symlin%20Injection_prntlbl.pdf (2005).
Ravussin, E. et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity 17, 1736–1743 (2009).
Chan, J. L. et al. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin. Endocrinol. 85, 137–149 (2016).
Roth, J. D., Erickson, M. R., Chen, S. & Parkes, D. G. GLP‐1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. Br. J. Pharmacol. 166, 121–136 (2012).
Mack, C. M. et al. Glucoregulatory effects and prolonged duration of action of davalintide: a novel amylinomimetic peptide. Diabetes Obes. Metab. 13, 1105–1113 (2011).
Biocentury. Amylin, Takeda discontinue davalintide. Biocentury www.biocentury.com/article/36923/amylin-takeda-discontinue-davalintide (2010).
Gydesen, S. et al. KBP-088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. Am. J. Physiol. Endocrinol. Metab. 310, E821–E827 (2016).
Gydesen, S. et al. A novel dual amylin and calcitonin receptor agonist, KBP-089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. Br. J. Pharmacol. 174, 591–602 (2017).
Mohamed, K. E. et al. The dual amylin and calcitonin receptor agonist KBP-336 elicits a unique combination of weight loss, antinociception and bone protection – a novel disease-modifying osteoarthritis drug. Arthritis Res. Ther. 26, 129 (2024).
Andreassen, K. V. et al. KBP-066A, a long-acting dual amylin and calcitonin receptor agonist, induces weight loss and improves glycemic control in obese and diabetic rats. Mol. Metab. 53, 101282 (2021).
Andreassen, K. V. et al. A novel oral dual amylin and calcitonin receptor agonist (KBP-042) exerts antiobesity and antidiabetic effects in rats. Am. J. Physiol. Endocrinol. Metab. 307, E24–E33 (2014).
Melson, E., Ashraf, U., Papamargaritis, D. & Davies, M. J. What is the pipeline for future medications for obesity? Int. J. Obes. 49, 433–451 (2025).
Manalac, T. AstraZeneca bolsters obesity pipeline with promising early data for candidates. BioSpace www.biospace.com/drug-development/astrazeneca-bolsters-obesity-pipeline-with-promising-early-data-for-candidates (2024).
Murray, A. et al. Amylin receptor (hAMY3R) agonists with improved chemical stability. International Patent WO2024003359A1 (2024).
World Health Organization. International nonproprietary names for pharmaceutical substances. WHO Drug Inf. 37, 436–437 (2023).
American Medical Association. Statement on a nonproprietary name adopted by the USAN Council: Eloralintide. AMA searchusan.ama-assn.org/usan/documentDownload?uri=/unstructured/binary/usan/eloralintide.pdf (2024).
Fletcher, M. M. et al. AM833 is a novel agonist of calcitonin family G protein-coupled receptors: pharmacological comparison with six selective and nonselective agonists. J. Pharmacol. Exp. Ther. 377, 417–440 (2021).
Kruse, T. et al. Development of cagrilintide, a long-acting amylin analogue. J. Med. Chem. 64, 11183–11194 (2021).
Larsen, A. T. et al. Does receptor balance matter? – comparing the efficacies of the dual amylin and calcitonin receptor agonists cagrilintide and KBP-336 on metabolic parameters in preclinical models. Biomed. Pharmacother. 156, 113842 (2022).
D’Ascanio, A. M., Mullally, J. A. & Frishman, W. H. Cagrilintide: a long-acting amylin analog for the treatment of obesity. Cardiol. Rev. 32, 83–90 (2024).
Henney, A. E., Wilding, J. P., Alam, U. & Cuthbertson, D. J. Obesity pharmacotherapy in older adults: a narrative review of evidence. Int. J. Obes. 49, 369–380 (2025).
Frias, J. P. et al. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 402, 720–730 (2023).
Kruse, T. H. et al. Co-agonists of the Glp-1 and amylin receptors. US Patent US20230331803A1 (2023).
Bower, R. L. et al. Molecular signature for receptor engagement in the metabolic peptide hormone amylin. ACS Pharmacol. Transl. Sci. 1, 32–49 (2018).
Trevaskis, J. L. et al. Improved glucose control and reduced body weight in rodents with dual mechanism of action peptide hybrids. PLoS ONE 8, e78154 (2013).
Sun, C. et al. Bifunctional PEGylated exenatide-amylinomimetic hybrids to treat metabolic disorders: an example of long-acting dual hormonal therapeutics. J. Med. Chem. 56, 9328–9341 (2013).
Haataja, L., Gurlo, T., Huang, C. J. & Butler, P. C. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 29, 303–316 (2008).
Noh, D., Bower, R. L., Hay, D. L., Zhyvoloup, A. & Raleigh, D. P. Analysis of amylin consensus sequences suggests that human amylin is not optimized to minimize amyloid formation and provides clues to factors that modulate amyloidogenicity. ACS Chem. Biol. 15, 1408–1416 (2020).
Westermark, P., Engstrom, U., Johnson, K. H., Westermark, G. T. & Betsholtz, C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc. Natl Acad. Sci. USA 87, 5036–5040 (1990).
Smaoui, M. R. & Waldispuhl, J. Complete characterization of the mutation landscape reveals the effect on amylin stability and amyloidogenicity. Proteins 83, 1014–1026 (2015).
Sakagashira, S. et al. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am. J. Pathol. 157, 2101–2109 (2000).
Lee, S. et al. The islet amyloid polypeptide (amylin) gene S20G mutation in Chinese subjects: evidence for associations with type 2 diabetes and cholesterol levels. Clin. Endocrinol. 54, 541–546 (2001).
Mendes, W. S., Franco, O. L., Alencar, S. A. & Porto, W. F. Structural effects driven by rare point mutations in amylin hormone, the type II diabetes-associated peptide. Biochim. Biophys. Acta Gen. Subj. 1865, 129935 (2021).
Wiltzius, J. J. W., Sievers, S. A., Sawaya, M. R. & Eisenberg, D. Atomic structures of IAPP (amylin) fusions suggest a mechanism for fibrillation and the role of insulin in the process. Protein Sci. 18, 1521–1530 (2009).
Poa, N., Cooper, G. & Edgar, P. Amylin gene promoter mutations predispose to type 2 diabetes in New Zealand Maori. Diabetologia 46, 574–578 (2003).
The 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Marmentini, C., Branco, R. C. S., Boschero, A. C. & Kurauti, M. A. Islet amyloid toxicity: from genesis to counteracting mechanisms. J. Cell Physiol. 237, 1119–1142 (2022).
Zhang, S., Liu, H., Yu, H. & Cooper, G. J. Fas-associated death receptor signaling evoked by human amylin in islet beta-cells. Diabetes 57, 348–356 (2008).
Cooper, G., Aitken, J. & Zhang, S. Is type 2 diabetes an amyloidosis and does it really matter (to patients)? Diabetologia 53, 1011–1016 (2010).
Zhang, S., Liu, J., Dragunow, M. & Cooper, G. J. Fibrillogenic amylin evokes islet β-cell apoptosis through linked activation of a caspase cascade and JNK1. J. Biol. Chem. 278, 52810–52819 (2003).
Abedini, A. et al. RAGE binds preamyloid IAPP intermediates and mediates pancreatic beta cell proteotoxicity. J. Clin. Invest. 128, 682–698 (2018).
Roesti, E. S. et al. Vaccination against amyloidogenic aggregates in pancreatic islets prevents development of type 2 diabetes mellitus. Vaccines 8, 116 (2020).
Vogt, A. C. S. et al. Anti-IAPP monoclonal antibody improves clinical symptoms in a mouse model of type 2 diabetes. Vaccines 9, 1316 (2021).
Wirth, F. et al. A human antibody against pathologic IAPP aggregates protects beta cells in type 2 diabetes models. Nat. Commun. 14, 6294 (2023).
Bram, Y. et al. Apoptosis induced by islet amyloid polypeptide soluble oligomers is neutralized by diabetes-associated specific antibodies. Sci. Rep. 4, 4267 (2014).
Wong, W. P. et al. Spontaneous diabetes in hemizygous human amylin transgenic mice that developed neither islet amyloid nor peripheral insulin resistance. Diabetes 57, 2737–2744 (2008).
Verchere, C. B., D’Alessio, D. A., Wang, S., Andrikopoulos, S. & Kahn, S. E. Transgenic overproduction of islet amyloid polypeptide (amylin) is not sufficient for islet amyloid formation. Horm. Metab. Res. 29, 311–316 (1997).
van Hulst, K. L. et al. Biologically active human islet amyloid polypeptide/amylin in transgenic mice. Eur. J. Endocrinol. 136, 107–113 (1997).
Yagui, K. et al. Formation of islet amyloid fibrils in beta-secretory granules of transgenic mice expressing human islet amyloid polypeptide/amylin. Eur. J. Endocrinol. 132, 487–496 (1995).
Aitken, J. F. et al. Tetracycline treatment retards the onset and slows the progression of diabetes in human amylin/islet amyloid polypeptide transgenic mice. Diabetes 59, 161–171 (2010).
Uhlen, M. et al. A proposal for validation of antibodies. Nat. Methods 13, 823–827 (2016).
Rees, T. A., Hendrikse, E. R., Hay, D. L. & Walker, C. S. Beyond CGRP: the calcitonin peptide family as targets for migraine and pain. Br. J. Pharmacol. 179, 381–399 (2022).
Russo, A. F. Overview of neuropeptides: awakening the senses? Headache 57, 37–46 (2017).
Rees, T. A., Hay, D. L. & Walker, C. S. Amylin antibodies frequently display cross-reactivity with CGRP: characterization of eight amylin antibodies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 320, R697–R703 (2021).
Ghanizada, H. et al. Amylin analog pramlintide induces migraine-like attacks in patients. Ann. Neurol. 89, 1157–1171 (2021).
Konarkowska, B., Aitken, J. F., Kistler, J., Zhang, S. & Cooper, G. J. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 273, 3614–3624 (2006).
Walsh, D. M., Klyubin, I., Fadeeva, J. V., Rowan, M. J. & Selkoe, D. J. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 30, 552–557 (2002).
Ke, P. C. et al. Implications of peptide assemblies in amyloid diseases. Chem. Soc. Rev. 46, 6492–6531 (2017).
Oskarsson, M. E. et al. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am. J. Pathol. 185, 834–846 (2015).
Jackson, K. et al. Amylin deposition in the brain: a second amyloid in Alzheimer disease? Ann. Neurol. 74, 517–526 (2013).
Libard, S. & Alafuzoff, I. Is islet amyloid polypeptide indeed expressed in the human brain? Neuropathol. Appl. Neurobiol. 49, e12917 (2023).
Hendrikse, E. R. et al. Characterization of antibodies against receptor activity-modifying protein 1 (RAMP1): a cautionary tale. Int. J. Mol. Sci. 23, 16035 (2022).
Eftekhari, S. et al. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 169, 683–696 (2010).
Acknowledgements
The authors acknowledge funding support from the New Zealand Ministry of Business, Innovation & Employment Hīkina Whakatutuki (UOAX1809; UOA24102).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. G.J.S.C., C.S.W., J.F.A. and S.Z. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.S.W. has received research support from AbbVie Inc. The other authors declare no other competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Thomas Lutz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Walker, C.S., Aitken, J.F., Vazhoor Amarsingh, G. et al. Amylin: emergent therapeutic opportunities in overweight, obesity and diabetes mellitus. Nat Rev Endocrinol (2025). https://doi.org/10.1038/s41574-025-01125-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41574-025-01125-9