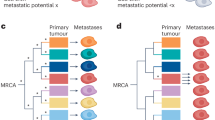
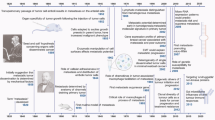
Abstract
Deciphering metastatic processes is crucial for understanding cancer progression and potential treatment options. Genetic studies of model systems engineered to mimic metastatic disease, including organoids, genetically engineered mice and human cell lines, have had an important role in shaping our understanding of the metastatic cascade and how it can be manipulated. More recently, advances in high-throughput sequencing have enabled human metastases to be studied at single-cell and single-nucleotide resolution, providing insights into metastatic evolution and phenotypes of both cancer cells and immune cells. However, human tissue studies are often correlative and descriptive, whereas experimental models are reductionistic by nature, meaning that individual results should be interpreted with caution. Crucially, these seemingly disparate branches of metastasis research can and should complement each other to strengthen and validate findings. Here we explore the synergies between model systems and sequencing studies and outline key areas that must be explored to improve our understanding of the metastatic process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Seyfried, T. N. & Huysentruyt, L. C. On the origin of cancer metastasis. Crit. Rev. Oncog. 18, 43–73 (2013).
Fares, J., Fares, M. Y., Khachfe, H. H., Salhab, H. A. & Fares, Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct. Target. Ther. 5, 28 (2020).
Nguyen, D. X., Bos, P. D. & Massagué, J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009).
Gupta, G. P. & Massagué, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Hapach, L. A., Mosier, J. A., Wang, W. & Reinhart-King, C. A. Engineered models to parse apart the metastatic cascade. npj Precis. Oncol. 3, 20 (2019).
Jandial, R. Metastatic Cancer: Clinical and Biological Perspectives (CRC Press, 2013).
Sajjad, H. et al. Cancer models in preclinical research: a chronicle review of advancement in effective cancer research. Anim. Model. Exp. Med. 4, 87–103 (2021).
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Langley, R. R. & Fidler, I. J. The seed and soil hypothesis revisited — the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 128, 2527–2535 (2011).
Al Bakir, M. et al. The evolution of non-small cell lung cancer metastases in TRACERx. Nature 616, 534–542 (2023). This study of a multiregional cohort of paired primary tumour–metastasis samples illustrates the evolutionary trajectories of NSCLC metastases and highlights the importance of extensive tumour sampling.
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017).
Robinson, D. R. et al. Integrative clinical genomics of metastatic cancer. Nature 548, 297–303 (2017).
Bertucci, F. et al. Genomic characterization of metastatic breast cancers. Nature 569, 560–564 (2019).
Angelova, M. et al. Evolution of metastases in space and time under immune selection. Cell 175, 751–765.e16 (2018).
Birkbak, N. J. & McGranahan, N. Cancer genome evolutionary trajectories in metastasis. Cancer Cell 37, 8–19 (2020).
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173, 581–594.e12 (2018).
Turajlic, S. & Swanton, C. Metastasis as an evolutionary process. Science 352, 169–175 (2016).
Hu, Z. & Curtis, C. Looking backward in time to define the chronology of metastasis. Nat. Commun. 11, 3213 (2020).
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5, 1164–1177 (2015).
Welch, D. R. & Hurst, D. R. Defining the hallmarks of metastasis. Cancer Res. 79, 3011–3027 (2019).
Stuelten, C. H., Parent, C. A. & Montell, D. J. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat. Rev. Cancer 18, 296–312 (2018).
Sherwood, D. R. & Sternberg, P. W. Anchor cell invasion into the vulval epithelium in C. elegans. Dev. Cell 5, 21–31 (2003).
Matus, D. Q. et al. Invasive cell fate requires G1 cell-cycle arrest and histone deacetylase-mediated changes in gene expression. Dev. Cell 35, 162–174 (2015).
Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016). This study uses multicolour lineage tracing in a mouse model of breast cancer, providing visual evidence for metastases that arose via polyclonal dissemination.
Konen, J. et al. Image-guided genomics of phenotypically heterogeneous populations reveals vascular signalling during symbiotic collective cancer invasion. Nat. Commun. 8, 15078 (2017).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400 (2007).
Noorani, A. et al. Genomic evidence supports a clonal diaspora model for metastases of esophageal adenocarcinoma. Nat. Genet. 52, 74–83 (2020).
Lee, J. E., Kim, K. T., Shin, S.-J., Cheong, J.-H. & Choi, Y. Y. Genomic and evolutionary characteristics of metastatic gastric cancer by routes. Br. J. Cancer 129, 672–682 (2023).
Nguyen, B. et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 185, 563–575.e11 (2022). A pan-cancer study of metastatic disease in more than 25,000 patients that emphasizes the role of chromosomal instability and identifies somatic alterations that are potentially important in driving metastasis.
Christensen, D. S. et al. Treatment represents a key driver of metastatic cancer evolution. Cancer Res. 82, 2918–2927 (2022).
Hu, Z., Li, Z., Ma, Z. & Curtis, C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat. Genet. 52, 701–708 (2020).
Martínez-Jiménez, F. et al. Pan-cancer whole-genome comparison of primary and metastatic solid tumours. Nature 618, 333–341 (2023).
Lengel, H. B. et al. Genomic mapping of metastatic organotropism in lung adenocarcinoma. Cancer Cell 41, 970–985.e3 (2023).
Tang, W.-F. et al. Timing and origins of local and distant metastases in lung cancer. J. Thorac. Oncol. 16, 1136–1148 (2021).
Biermann, J. et al. Dissecting the treatment-naive ecosystem of human melanoma brain metastasis. Cell 185, 2591–2608.e30 (2022).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Priestley, P. et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575, 210–216 (2019).
Frankell, A. M. et al. The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature 616, 525–533 (2023).
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376, 2109–2121 (2017).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Hu, Z. et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 51, 1113–1122 (2019).
Litchfield, K. et al. Representative sequencing: unbiased sampling of solid tumor tissue. Cell Rep. 31, 107550 (2020).
Tomasetti, C., Vogelstein, B. & Parmigiani, G. Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc. Natl Acad. Sci. USA 110, 1999–2004 (2013).
Lim, C.-H. et al. The chronological sequence of somatic mutations in early gastric carcinogenesis inferred from multiregion sequencing of gastric adenomas. Oncotarget 7, 39758–39767 (2016).
Lee, S. H., Yoo, J., Song, Y. S., Lim, C.-H. & Kim, T.-M. Mutation analysis of colorectal and gastric carcinomas originating from adenomas: insights into genomic evolution associated with malignant progression. Cancers 12, 325 (2020).
Jin, X. et al. A metastasis map of human cancer cell lines. Nature 588, 331–336 (2020).
Allen, T. M. et al. Humanized immune system mouse models: progress, challenges and opportunities. Nat. Immunol. 20, 770–774 (2019).
Huebner, A., Dietzen, M. & McGranahan, N. SnapShot: tumor evolution. Cell 184, 1650–1650.e1 (2021).
Grigoriadis, K. et al. CONIPHER: a computational framework for scalable phylogenetic reconstruction with error correction. Nat. Protoc. 19, 159–183 (2024).
Venet, D. et al. Phylogenetic reconstruction of breast cancer reveals two routes of metastatic dissemination associated with distinct clinical outcome. EBioMedicine 56, 102793 (2020).
Andersson, N., Chattopadhyay, S., Valind, A., Karlsson, J. & Gisselsson, D. DEVOLUTION — a method for phylogenetic reconstruction of aneuploid cancers based on multiregional genotyping data. Commun. Biol. 4, 1103 (2021).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
El-Kebir, M., Satas, G. & Raphael, B. J. Inferring parsimonious migration histories for metastatic cancers. Nat. Genet. 50, 718–726 (2018). This study introduces a computational algorithm that infers metastatic seeding and migration patterns; it suggests that polyclonal dissemination might be over-reported upon undersampling.
Brown, D. et al. Phylogenetic analysis of metastatic progression in breast cancer using somatic mutations and copy number aberrations. Nat. Commun. 8, 14944 (2017).
Hoadley, K. A. et al. Tumor evolution in two patients with basal-like breast cancer: a retrospective genomics study of multiple metastases. PLoS Med. 13, e1002174 (2016).
Abbosh, C. et al. Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 616, 553–562 (2023).
Reeves, M. Q., Kandyba, E., Harris, S., Del Rosario, R. & Balmain, A. Multicolour lineage tracing reveals clonal dynamics of squamous carcinoma evolution from initiation to metastasis. Nat. Cell Biol. 20, 699–709 (2018).
Maddipati, R. & Stanger, B. Z. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 5, 1086–1097 (2015). Using a Cas9-based lineage tracer and single-cell RNA sequencing, this study reveals complex seeding and migration patterns of human lung cancer in a mouse xenograft model.
Spanjaard, B. et al. Simultaneous lineage tracing and cell-type identification using CRISPR–Cas9-induced genetic scars. Nat. Biotechnol. 36, 469–473 (2018).
Tang, Y. J. et al. Tracing tumor evolution in sarcoma reveals clonal origin of advanced metastasis. Cell Rep. 28, 2837–2850.e5 (2019).
Simeonov, K. P. et al. Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states. Cancer Cell 39, 1150–1162.e9 (2021).
Quinn, J. J. et al. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts. Science 371, eabc1944 (2021).
Karlsson, K. et al. Deterministic evolution and stringent selection during preneoplasia. Nature 618, 383–393 (2023).
Lambert, A. W. & Weinberg, R. A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 21, 325–338 (2021).
Xu, J., Lamouille, S. & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 (2009).
Kim, B. N. et al. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci. Rep. 10, 10597 (2020).
Cano, A. et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000).
Park, S.-H. et al. Particulate matter promotes cancer metastasis through increased HBEGF expression in macrophages. Exp. Mol. Med. 54, 1901–1912 (2022).
Richters, A. & Kuraitis, K. Air pollutants and the facilitation of cancer metastasis. Environ. Health Perspect. 52, 165–168 (1983).
Hill, W. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616, 159–167 (2023).
Zheng, X. et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 (2015).
Fischer, K. R. et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476 (2015).
Pastushenko, I. et al. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468 (2018). This study shows that multiple tumour cell subtypes, including those in non-polarized hybrid states, exist during EMT, with different levels of various factors being associated with metastatic aggression.
Pal, A., Barrett, T. F., Paolini, R., Parikh, A. & Puram, S. V. Partial EMT in head and neck cancer biology: a spectrum instead of a switch. Oncogene 40, 5049–5065 (2021).
Puram, S. V. et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624.e24 (2017).
Roshanzamir, F., Robinson, J. L., Cook, D., Karimi-Jafari, M. H. & Nielsen, J. Metastatic triple negative breast cancer adapts its metabolism to destination tissues while retaining key metabolic signatures. Proc. Natl Acad. Sci. USA 119, e2205456119 (2022).
Pavlič, A., Urh, K., Štajer, K., Boštjančič, E. & Zidar, N. Epithelial–mesenchymal transition in colorectal carcinoma: comparison between primary tumor, lymph node and liver metastases. Front. Oncol. 11, 662806 (2021).
Kamal, Y., Schmit, S. L., Hoehn, H. J., Amos, C. I. & Frost, H. R. Transcriptomic differences between primary colorectal adenocarcinomas and distant metastases reveal metastatic colorectal cancer subtypes. Cancer Res. 79, 4227–4241 (2019).
Lili, L. N. et al. Molecular profiling supports the role of epithelial-to-mesenchymal transition (EMT) in ovarian cancer metastasis. J. Ovarian Res. 6, 49 (2013).
Winkler, J. et al. Single-cell analysis of breast cancer metastasis reveals epithelial-mesenchymal plasticity signatures associated with poor outcomes. J. Clin. Invest. 134, e164227 (2024).
Moorman, A. R. et al. Progressive plasticity during colorectal cancer metastasis. Nature 637, 947–954 (2025). This study reports a conserved, progenitor-like, plastic tumour cell state that can differentiate into multiple lineages of cells that are enriched in colorectal cancer metastases.
Gonzalez, H. et al. Cellular architecture of human brain metastases. Cell 185, 729–745.e20 (2022).
Laughney, A. M. et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat. Med. 26, 259–269 (2020).
Nam, A. S. et al. Somatic mutations and cell identity linked by Genotyping of Transcriptomes. Nature 571, 355–360 (2019).
Monteran, L., Zait, Y. & Erez, N. It’s all about the base: stromal cells are central orchestrators of metastasis. Trends Cancer 10, 208–229 (2024).
Bussard, K. M., Mutkus, L., Stumpf, K., Gomez-Manzano, C. & Marini, F. C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 18, 84 (2016).
Guo, S. & Deng, C.-X. Effect of stromal cells in tumor microenvironment on metastasis initiation. Int. J. Biol. Sci. 14, 2083–2093 (2018).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Asif, P. J., Longobardi, C., Hahne, M. & Medema, J. P. The role of cancer-associated fibroblasts in cancer invasion and metastasis. Cancers 13, 4720 (2021).
Wang, L. et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 8, 76116–76128 (2017).
Cords, L. et al. Cancer-associated fibroblast phenotypes are associated with patient outcome in non-small cell lung cancer. Cancer Cell 42, 396–412.e5 (2024). Using spatially resolved, single-cell, imaging mass cytometry, this study identifies distinct CAF populations that are associated with various determinants of patient outcome.
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
Enfield, K. S. S. et al. Spatial architecture of myeloid and T cells orchestrates immune evasion and clinical outcome in lung cancer. Cancer Discov. 14, 1018–1047 (2024).
Jia, C.-C. et al. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS ONE 8, e63243 (2013).
Zhang, L. et al. Cancer-associated fibroblast-related gene signatures predict survival and drug response in patients with colorectal cancer. Front. Genet. 13, 1054152 (2022).
Chen, Z. et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 11, 5077 (2020).
Erez, N., Truitt, M., Olson, P., Arron, S. T. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17, 135–147 (2010).
Erez, N., Glanz, S., Raz, Y., Avivi, C. & Barshack, I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 437, 397–402 (2013).
Cords, L. et al. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat. Commun. 14, 4294 (2023).
Lambrechts, D. et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 24, 1277–1289 (2018).
Lavie, D., Ben-Shmuel, A., Erez, N. & Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 3, 793–807 (2022).
Luo, H. et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 13, 6619 (2022). This study highlights the heterogeneity of CAFs and their plasticity across cancer types, and suggests evolutionary trajectories that could lead to distinct CAF states.
Shih, A. J. et al. Identification of grade and origin specific cell populations in serous epithelial ovarian cancer by single cell RNA-seq. PLoS ONE 13, e0206785 (2018).
Gong, Z. et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity 55, 1483–1500.e9 (2022).
Zeng, H. et al. Cancer-associated fibroblasts facilitate premetastatic niche formation through lncRNA SNHG5-mediated angiogenesis and vascular permeability in breast cancer. Theranostics 12, 7351–7370 (2022).
Patras, L., Shaashua, L., Matei, I. & Lyden, D. Immune determinants of the pre-metastatic niche. Cancer Cell 41, 546–572 (2023).
Wang, Z. et al. Metastasis-associated fibroblasts: an emerging target for metastatic cancer. Biomark. Res. 9, 47 (2021).
Zheng, Z. et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat. Commun. 12, 6202 (2021).
Suresh, R. & Diaz, R. J. The remodelling of actin composition as a hallmark of cancer. Transl. Oncol. 14, 101051 (2021).
Yamaguchi, H. & Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773, 642–652 (2007).
Swierczak, A., Mouchemore, K. A., Hamilton, J. A. & Anderson, R. L. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 34, 735–751 (2015).
Xiong, S., Dong, L. & Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 14, 173 (2021).
Galdiero, M. R. et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 218, 1402–1410 (2013).
Giese, M. A., Hind, L. E. & Huttenlocher, A. Neutrophil plasticity in the tumor microenvironment. Blood 133, 2159–2167 (2019).
Jia, J. et al. Neutrophils in the premetastatic niche: key functions and therapeutic directions. Mol. Cancer 23, 200 (2024).
Kaltenmeier, C., Simmons, R. L., Tohme, S. & Yazdani, H. O. Neutrophil extracellular traps (NETs) in cancer metastasis. Cancers 13, 6131 (2021).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015). This study shows the role of neutrophils in establishing a pre-metastatic niche in the lung in breast cancer, by means such as the selective proliferation of cells with high metastasis-initiating potential.
Lee, W. et al. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 216, 176–194 (2019).
Neophytou, C. M., Panagi, M., Stylianopoulos, T. & Papageorgis, P. The role of tumor microenvironment in cancer metastasis: molecular mechanisms and therapeutic opportunities. Cancers 13, 2053 (2021).
Neophytou, C. M. et al. The role of tumor-associated myeloid cells in modulating cancer therapy. Front. Oncol. 10, 899 (2020).
Pan, Y., Yu, Y., Wang, X. & Zhang, T. Corrigendum: tumor-associated macrophages in tumor immunity. Front. Immunol. 12, 775758 (2021).
Lin, Y., Xu, J. & Lan, H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 12, 76 (2019).
Ma, R.-Y., Black, A. & Qian, B.-Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563 (2022).
Wang, J., Zhu, N., Su, X., Gao, Y. & Yang, R. Novel tumor-associated macrophage populations and subpopulations by single cell RNA sequencing. Front. Immunol. 14, 1264774 (2023).
Wyckoff, J. B. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007).
Tang, J. et al. The heterogeneity of tumour-associated macrophages contributes to the clinical outcomes and indications for immune checkpoint blockade in colorectal cancer patients. Immunobiology 229, 152805 (2024). This study identifies subtypes of TAMs and suggests that TAMs that reside at the tumour border are more likely to be responsive to immunotherapy because they have more genomic disruption.
Su, S. et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 25, 605–620 (2014).
Comito, G. et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 33, 2423–2431 (2014).
Kim, J. H. et al. The role of myofibroblasts in upregulation of S100A8 and S100A9 and the differentiation of myeloid cells in the colorectal cancer microenvironment. Biochem. Biophys. Res. Commun. 423, 60–66 (2012).
Mao, X. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer 20, 131 (2021).
Davidson, S. et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 31, 107628 (2020).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563, 347–353 (2018).
Dimitrov, D. et al. LIANA+ provides an all-in-one framework for cell-cell communication inference. Nat. Cell Biol. 26, 1613–1622 (2024).
Karimi, E. et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 614, 555–563 (2023).
Yu, M., Stott, S., Toner, M., Maheswaran, S. & Haber, D. A. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 192, 373–382 (2011).
Mohammed, S. I., Torres-Luquis, O., Walls, E. & Lloyd, F. Lymph-circulating tumor cells show distinct properties to blood-circulating tumor cells and are efficient metastatic precursors. Mol. Oncol. 13, 1400–1418 (2019).
Nasr, M. M. & Lynch, C. C. How circulating tumor cluster biology contributes to the metastatic cascade: from invasion to dissemination and dormancy. Cancer Metastasis Rev. 42, 1133–1146 (2023).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014). This study shows that CTC clusters arise from the heterogeneous primary tumour rather than through intravascular aggregation, and have greater metastatic potential than singular CTCs.
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Ting, D. T. et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 8, 1905–1918 (2014).
Seo, J. et al. Plasticity of circulating tumor cells in small cell lung cancer. Sci. Rep. 13, 11775 (2023).
Cheng, Y.-H. et al. Hydro-Seq enables contamination-free high-throughput single-cell RNA-sequencing for circulating tumor cells. Nat. Commun. 10, 2163 (2019).
Barrière, G., Riouallon, A., Renaudie, J., Tartary, M. & Rigaud, M. Mesenchymal and stemness circulating tumor cells in early breast cancer diagnosis. BMC Cancer 12, 114 (2012).
Papadaki, M. A. et al. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol. Cancer Ther. 18, 437–447 (2019).
Morel, A.-P. et al. Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS ONE 3, e2888 (2008).
Negishi, R. et al. Transcriptomic profiling of single circulating tumor cells provides insight into human metastatic gastric cancer. Commun. Biol. 5, 20 (2022).
Powell, A. A. et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE 7, e33788 (2012).
Oulhen, M. et al. Circulating tumor cell copy-number heterogeneity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. npj Precis. Oncol. 5, 67 (2021).
Carter, L. et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 23, 114–119 (2017).
Paoletti, C. et al. Comprehensive mutation and copy number profiling in archived circulating breast cancer tumor cells documents heterogeneous resistance mechanisms. Cancer Res. 78, 1110–1122 (2018).
Moose, D. L. et al. Cancer cells resist mechanical destruction in circulation via RhoA/actomyosin-dependent mechano-adaptation. Cell Rep. 30, 3864–3874.e6 (2020).
Regmi, S., Fu, A. & Luo, K. Q. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci. Rep. 7, 39975 (2017).
Mammadova-Bach, E. et al. Platelet integrin α6β1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI Insight 1, e88245 (2016). This study shows the functional role of the tumour-resident microbiota in facilitating metastatic spread by reducing fluid shear stress during circulation.
Fu, A. et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 185, 1356–1372.e26 (2022).
Xin, Y., Li, K., Yang, M. & Tan, Y. Fluid shear stress induces EMT of circulating tumor cells via JNK signaling in favor of their survival during hematogenous dissemination. Int. J. Mol. Sci. 21, 8115 (2020).
Gaikwad, A. et al. Aerogenous metastases: a potential game changer in the diagnosis and management of primary lung adenocarcinoma. AJR Am. J. Roentgenol. 203, W570–W582 (2014).
Karasaki, T. et al. Evolutionary characterization of lung adenocarcinoma morphology in TRACERx. Nat. Med. 29, 833–845 (2023). This study reports evidence that supports the hypothesis of metastatic spread through the airspaces; such tumours were associated with poor prognosis.
Giancotti, F. G. Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013).
Karrison, T. G., Ferguson, D. J. & Meier, P. Dormancy of mammary carcinoma after mastectomy. J. Natl Cancer Inst. 91, 80–85 (1999).
Montagner, M. & Sahai, E. In vitro models of breast cancer metastatic dormancy. Front. Cell Dev. Biol. 8, 37 (2020).
Mahmoud, A. & Ganesh, K. Mouse models of metastasis and dormancy. Cold Spring Harb. Perspect. Med. 14, a041386 (2024).
Turrell, F. K. et al. Age-associated microenvironmental changes highlight the role of PDGF-C in ER+ breast cancer metastatic relapse. Nat. Cancer 4, 468–484 (2023). This study illustrates, in mice and human cohort data, the importance of PDGF-C in the often-observed delayed metastatic relapse of oestrogen receptor-positive breast cancer.
Malanchi, I. et al. An extramedullary bone model to study metastatic dormancy reveals a piecemeal model for metastatic reactivation. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-3173228/v1 (2023).
Izumchenko, E. et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann. Oncol. 28, 2595–2605 (2017).
Byrne, A. T. et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 17, 254–268 (2017).
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21, 1318–1325 (2015).
Hynds, R. E. et al. Representation of genomic intratumor heterogeneity in multi-region non-small cell lung cancer patient-derived xenograft models. Nat. Commun. 15, 4653 (2024).
Hessey, S., Fessas, P., Zaccaria, S., Jamal-Hanjani, M. & Swanton, C. Insights into the metastatic cascade through research autopsies. Trends Cancer 9, 490–502 (2023).
Zhang, Y. et al. Single-cell RNA sequencing reveals that the immunosuppression landscape induced by chronic stress promotes colorectal cancer metastasis. Heliyon 10, e23552 (2024).
Cheng, V. et al. Colorectal cancer and onset of anxiety and depression: a systematic review and meta-analysis. Curr. Oncol. 29, 8751–8766 (2022).
Acknowledgements
The authors thank K. Grimes, M. Zagorulya and A. Huebner for their insightful comments and discussions during the writing of this review. M.M.L. is supported by the Rosetrees Trust. C.S. is a Royal Society Napier Research Professor (RSRP\R\210001). His work is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (CRUK) (CC2041), the UK Medical Research Council (CC2041) and the Wellcome Trust (CC2041). N.M. is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (211179/Z/18/Z). He also receives funding from CRUK, the Rosetrees Trust and the NIHR BRC at University College London Hospitals.
Author information
Authors and Affiliations
Contributions
M.M.L. and N.M. researched data for the article. M.M.L. and N.M. contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
C.S. acknowledges grants from AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Pfizer, Roche-Ventana, Invitae (previously Archer Dx Inc. — collaboration in minimal residual disease sequencing technologies), Ono Pharmaceutical, and Personalis. He is Chief Investigator for the AstraZeneca MeRmaiD 1 and 2 clinical trials and is the Steering Committee Chair. He is also Co-Chief Investigator of the NHS Galleri trial funded by GRAIL and a paid member of GRAIL’s Scientific Advisory Board (SAB). He receives consultant fees from Achilles Therapeutics (also a SAB member), Bicycle Therapeutics (also a SAB member), Genentech, Medicxi, China Innovation Centre of Roche (CICoR; formerly Roche Innovation Centre, Shanghai), Metabomed (until July 2022), Relay Therapeutics SAB member, Saga Diagnostics SAB member and the Sarah Cannon Research Institute. C.S. has received honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Illumina, MSD, Novartis, Pfizer and Roche-Ventana. C.S. has previously held stock options in Apogen Biotechnologies and GRAIL, and currently has stock options in Epic Bioscience, Bicycle Therapeutics, Relay Therapeutics, and has stock options and is co-founder of Achilles Therapeutics. C.S. declares a patent application for methods to detect lung cancer (PCT/US2017/028013); targeting neoantigens (PCT/EP2016/059401); identifying patient response to immune checkpoint blockade (PCT/EP2016/071471); methods for lung cancer detection (US20190106751A1); identifying patients who respond to cancer treatment (PCT/GB2018/051912); determining HLA loss of heterozygosity (PCT/GB2018/052004); predicting survival rates of patients with cancer (PCT/GB2020/050221); and methods and systems for tumour monitoring (PCT/EP2022/077987). C.S. is an inventor on a European patent application (PCT/GB2017/053289) relating to assay technology to detect tumour recurrence. This patent has been licensed to a commercial entity and under their terms of employment C.S. is due a revenue share of any revenue generated from such license(s). N.M. has stock options in and has consulted for Achilles Therapeutics and holds a European patent in determining HLA loss of heterozygosity (PCT/GB2018/052004), a patent pending in determining HLA disruption (PCT/EP2023/059039), and is a co-inventor on a patent to identify responders to cancer treatment (PCT/GB2018/051912). M.M.L. declares no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Zheng Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Anoikis
-
A form of apoptosis that occurs when cells detach from the extracellular matrix and/or neighbouring cells.
- Basement membrane
-
A thin and pliable layer of extracellular matrix that lines tissue, providing structural support and separating cell types. Also known as the basal lamina.
- Circulating tumour DNA
-
Fragments of DNA from tumour cells that can be detected in the bloodstream.
- Confetti mouse
-
A genetically engineered mouse model that has a four-colour cassette that causes cells to be labelled randomly, allowing multicolour visualization.
- Contingent evolution
-
Evolution shaped by chance-based events, such as mutations.
- Convergent evolution
-
Evolution of separate lineages in response to similar environmental pressures, resulting in analogous phenotypes.
- Epithelial–mesenchymal transition
-
(EMT). The process by which an epithelial cell acquires mesenchymal characteristics, thereby increasing its invasiveness and migratory propensity towards metastasis.
- Invadopodia
-
Finger-like protrusions of the plasma membrane of a cell that facilitate cell migration.
- Matrix metalloproteinases
-
(MMPs). A family of proteolytic enzymes found in the extracellular matrix.
- Mesenchymal–epithelial transition
-
The reverse process of epithelial–mesenchymal transition, typically occurring in the later stages of metastasis (for example, during colonization at a site distant from the primary tumour), whereby a migrating tumour cell with mesenchymal properties re-acquires epithelial characteristics.
- Neoadjuvant therapy
-
Treatment provided before a main treatment. For cancer, examples include chemotherapy and hormone therapy, which are often used to shrink a tumour before surgery.
- Pre-metastatic niche
-
An environment distant from the primary tumour site that is primed for the colonization and growth of a tumour metastasis.
- Triple-negative breast cancer
-
A fast-growing and invasive type of breast cancer lacking expression of HER2 and oestrogen and progesterone receptors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leung, M.M., Swanton, C. & McGranahan, N. Integrating model systems and genomic insights to decipher mechanisms of cancer metastasis. Nat Rev Genet 26, 494–505 (2025). https://doi.org/10.1038/s41576-025-00825-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-025-00825-2