Abstract
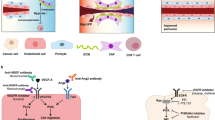
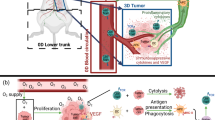
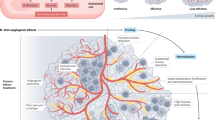
The formation of new blood vessels — known as angiogenesis — is essential for the growth and spread of solid tumours. It is promoted by the hypoxic conditions that develop in growing tumours and drive the expression of pro-angiogenic growth factors by tumour cells and various stromal cells. However, the tumour-associated vasculature (TAV) generated by angiogenesis is abnormal and is a key barrier to T cell entry into tumours. Moreover, the TAV creates a hostile microenvironment owing to an accumulation of suppressive immune cells, hypoxic and acidic conditions, and high interstitial pressure, which all limit the function and survival of effector T cells. Here, we present the mechanisms of T cell migration into tumours, including via high endothelial venules, and the importance of tertiary lymphoid structures, which function as privileged sites for antigen presentation, activation and co-stimulation of T cells, for mounting effective antitumour immunity. We describe how the tumour vasculature limits antitumour T cell responses and how T cell responses could be improved by therapeutic targeting of the TAV. In particular, the use of combination therapies that aim to normalize tumour blood vessels, favourably reprogramme endogenous immunity, and support T cell trafficking, function and persistence will be key to improving clinical responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anderson, N. M. & Simon, M. C. The tumor microenvironment. Curr. Biol. 30, R921–R925 (2020).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004).
Weis, S. M. & Cheresh, D. A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17, 1359–1370 (2011).
Lanitis, E., Irving, M. & Coukos, G. Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 33, 55–63 (2015).
Sarnaik, A. A., Hwu, P., Mule, J. J. & Pilon-Thomas, S. Tumor-infiltrating lymphocytes: a new hope. Cancer Cell 42, 1315–1318 (2024).
Giordano Attianese, G. M. P., Ash, S. & Irving, M. Coengineering specificity, safety, and function into T cells for cancer immunotherapy. Immunol. Rev. 320, 166–198 (2023).
Rockwell, S., Dobrucki, I. T., Kim, E. Y., Marrison, S. T. & Vu, V. T. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr. Mol. Med. 9, 442–458 (2009).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Chouaib, S., Noman, M. Z., Kosmatopoulos, K. & Curran, M. A. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 36, 439–445 (2017).
Gimbrone, M. A. Jr., Leapman, S. B., Cotran, R. S. & Folkman, J. Tumor dormancy in vivo by prevention of neovascularization. J. Exp. Med. 136, 261–276 (1972).
Forster, J. C., Harriss-Phillips, W. M., Douglass, M. J. & Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 5, 21–32 (2017).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Welti, J., Loges, S., Dimmeler, S. & Carmeliet, P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Invest. 123, 3190–3200 (2013).
McDonald, D. M. & Baluk, P. Significance of blood vessel leakiness in cancer. Cancer Res. 62, 5381–5385 (2002).
Hashizume, H. et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 156, 1363–1380 (2000).
Nagy, J. A. & Dvorak, H. F. Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin. Exp. Metastasis 29, 657–662 (2012).
De Palma, M. & Hanahan, D. Milestones in tumor vascularization and its therapeutic targeting. Nat. Cancer 5, 827–843 (2024).
Vuillefroy de Silly, R., Pericou, L., Seijo, B., Crespo, I. & Irving, M. Acidity suppresses CD8 + T-cell function by perturbing IL-2, mTORC1, and c-Myc signaling. EMBO J. 43, 4922-4953 (2024).
Dudley, A. C. & Griffioen, A. W. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis 26, 313–347 (2023).
Baeriswyl, V. & Christofori, G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 19, 329–337 (2009).
Semenza, G. L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 9, 47–71 (2014).
Vigano, S. et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front. Immunol. 10, 925 (2019).
Motz, G. T. & Coukos, G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat. Rev. Immunol. 11, 702–711 (2011).
Ma, R. Y., Black, A. & Qian, B. Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563 (2022).
Lapenna, A., De Palma, M. & Lewis, C. E. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 18, 689–702 (2018).
Wang, S. et al. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 8, 31 (2024).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020).
Mazzieri, R. et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19, 512–526 (2011).
Coffelt, S. B. et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J. Immunol. 186, 4183–4190 (2011).
Coffelt, S. B. et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 70, 5270–5280 (2010).
Turrini, R. et al. TIE-2 expressing monocytes in human cancers. Oncoimmunology 6, e1303585 (2017).
Barleon, B. et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87, 3336–3343 (1996).
McRitchie, B. R. & Akkaya, B. Exhaust the exhausters: targeting regulatory T cells in the tumor microenvironment. Front. Immunol. 13, 940052 (2022).
Sun, W. et al. A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. Br. J. Cancer 117, 1631–1643 (2017).
Komi, D. E. A. & Redegeld, F. A. Role of mast cells in shaping the tumor microenvironment. Clin. Rev. Allergy Immunol. 58, 313–325 (2020).
Hedrick, C. C. & Malanchi, I. Neutrophils in cancer: heterogeneous and multifaceted. Nat. Rev. Immunol. 22, 173–187 (2022).
Vetsika, E. K., Koukos, A. & Kotsakis, A. Myeloid-derived suppressor cells: major figures that shape the immunosuppressive and angiogenic network in cancer. Cells 8, 1647 (2019).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Kanzaki, R. & Pietras, K. Heterogeneity of cancer-associated fibroblasts: opportunities for precision medicine. Cancer Sci. 111, 2708–2717 (2020).
Jain, S. et al. Single-cell RNA sequencing and spatial transcriptomics reveal cancer-associated fibroblasts in glioblastoma with protumoral effects. J. Clin. Invest. 133, e147087 (2023).
Bartoschek, M. et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 9, 5150 (2018).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Elices, M. J. et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 60, 577–584 (1990).
Bridgeman, V. L. et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J. Pathol. 241, 362–374 (2017).
Teuwen, L. A. et al. Tumor vessel co-option probed by single-cell analysis. Cell Rep. 35, 109253 (2021).
Leenders, W. P. et al. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin. Cancer Res. 10, 6222–6230 (2004).
Kuczynski, E. A. & Reynolds, A. R. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis 23, 55–74 (2020).
Rada, M., Hassan, N., Lazaris, A. & Metrakos, P. The molecular mechanisms underlying neutrophil infiltration in vessel co-opting colorectal cancer liver metastases. Front. Oncol. 12, 1004793 (2022).
Zeng, Q. et al. Understanding tumour endothelial cell heterogeneity and function from single-cell omics. Nat. Rev. Cancer 23, 544–564 (2023).
Goveia, J. et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell 37, 21–36.e13 (2020).
Wenes, M. et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. 24, 701–715 (2016).
Opzoomer, J. W. et al. Macrophages orchestrate the expansion of a proangiogenic perivascular niche during cancer progression. Sci. Adv. 7, eabg9518 (2021).
De Palma, M. et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226 (2005).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Sackstein, R., Schatton, T. & Barthel, S. R. T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Invest. 97, 669–697 (2017).
Lawrence, M. B., Kansas, G. S., Kunkel, E. J. & Ley, K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E). J. Cell Biol. 136, 717–727 (1997).
Hahn, C. & Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 10, 53–62 (2009).
Lotscher, J. et al. Magnesium sensing via LFA-1 regulates CD8+ T cell effector function. Cell 185, 585–602.e29 (2022).
Rothlein, R., Dustin, M. L., Marlin, S. D. & Springer, T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 137, 1270–1274 (1986).
Osborn, L. et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59, 1203–1211 (1989).
Pober, J. S. et al. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J. Immunol. 137, 1893–1896 (1986).
Griffioen, A. W., Damen, C. A., Martinotti, S., Blijham, G. H. & Groenewegen, G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 56, 1111–1117 (1996).
Griffioen, A. W., Damen, C. A., Blijham, G. H. & Groenewegen, G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 88, 667–673 (1996).
Facciabene, A. et al. Local endothelial complement activation reverses endothelial quiescence, enabling T-cell homing, and tumor control during T-cell immunotherapy. Oncoimmunology 6, e1326442 (2017).
Girard, J. P. & Springer, T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995).
Engelhard, V. H. et al. Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. J. Immunol. 200, 432–442 (2018).
Vella, G., Hua, Y. & Bergers, G. High endothelial venules in cancer: regulation, function, and therapeutic implication. Cancer Cell 41, 527–545 (2023).
Streeter, P. R., Rouse, B. T. & Butcher, E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
Martinet, L. et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 71, 5678–5687 (2011).
Lee, M. et al. Transcriptional programs of lymphoid tissue capillary and high endothelium reveal control mechanisms for lymphocyte homing. Nat. Immunol. 15, 982–995 (2014).
Colbeck, E. J. et al. Treg depletion licenses T cell-driven HEV neogenesis and promotes tumor destruction. Cancer Immunol. Res. 5, 1005–1015 (2017).
Hindley, J. P. et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res. 72, 5473–5482 (2012).
Tsuboi, K. et al. Role of high endothelial venule-expressed heparan sulfate in chemokine presentation and lymphocyte homing. J. Immunol. 191, 448–455 (2013).
Melssen, M. M., Sheybani, N. D., Leick, K. M. & Slingluff, C. L. Jr. Barriers to immune cell infiltration in tumors. J. Immunother. Cancer 11, e006401 (2023).
Peske, J. D. et al. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat. Commun. 6, 7114 (2015).
Ruddle, N. H. High endothelial venules and lymphatic vessels in tertiary lymphoid organs: characteristics, functions, and regulation. Front. Immunol. 7, 491 (2016).
Sautes-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Martinet, L. et al. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 1, 829–839 (2012).
Hua, Y. et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1+ T lymphocyte niches through a feed-forward loop. Cancer Cell 40, 1600–1618.e10 (2022).
Asrir, A. et al. Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell 40, 318–334.e9 (2022).
Colbeck, E. J., Ager, A., Gallimore, A. & Jones, G. W. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front. Immunol. 8, 1830 (2017).
Schumacher, T. N. & Thommen, D. S. Tertiary lymphoid structures in cancer. Science 375, eabf9419 (2022).
de Chaisemartin, L. et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 71, 6391–6399 (2011).
Goc, J. et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 74, 705–715 (2014).
Vella, G., Guelfi, S. & Bergers, G. High endothelial venules: a vascular perspective on tertiary lymphoid structures in cancer. Front. Immunol. 12, 736670 (2021).
Bouzin, C., Brouet, A., De Vriese, J., Dewever, J. & Feron, O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J. Immunol. 178, 1505–1511 (2007).
Buckanovich, R. J. et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 14, 28–36 (2008).
Ma, W. et al. Targeting PAK4 to reprogram the vascular microenvironment and improve CAR-T immunotherapy for glioblastoma. Nat. Cancer 2, 83–97 (2021).
Hellebrekers, D. M. et al. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res. 66, 10770–10777 (2006).
Kim, D. J. et al. Priming a vascular-selective cytokine response permits CD8+ T-cell entry into tumors. Nat. Commun. 14, 2122 (2023).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Karikoski, M. et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clin. Cancer Res. 20, 6452–6464 (2014).
Kabir, A. U. et al. Dual role of endothelial Myct1 in tumor angiogenesis and tumor immunity. Sci. Transl. Med. 13, eabb6731 (2021).
Guelfi, S., Hodivala-Dilke, K. & Bergers, G. Targeting the tumour vasculature: from vessel destruction to promotion. Nat. Rev. Cancer 24, 655–675 (2024).
Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001).
Martin, J. D., Seano, G. & Jain, R. K. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu. Rev. Physiol. 81, 505–534 (2019).
Yang, T. et al. Vascular normalization: a new window opened for cancer therapies. Front. Oncol. 11, 719836 (2021).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Ruegg, C. & Mutter, N. Anti-angiogenic therapies in cancer: achievements and open questions. Bull. Cancer 94, 753–762 (2007).
Plate, K. H., Breier, G., Weich, H. A. & Risau, W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359, 845–848 (1992).
Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475, 226–230 (2011).
Willett, C. G. et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 10, 145–147 (2004).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Secondini, C. et al. Arginase inhibition suppresses lung metastasis in the 4T1 breast cancer model independently of the immunomodulatory and anti-metastatic effects of VEGFR-2 blockade. Oncoimmunology 6, e1316437 (2017).
Tada, Y. et al. Targeting VEGFR2 with ramucirumab strongly impacts effector/activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J. Immunother. Cancer 6, 106 (2018).
Arance, A. et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J. Clin. Oncol. 41, 75–85 (2023).
Merck and Eisai provide update on phase 3 trials of KEYTRUDA® (pembrolizumab) plus LENVIMA® (lenvatinib) in certain patients with advanced melanoma (LEAP-003) and metastatic colorectal cancer (LEAP-017). https://www.eisai.com/news/2023/pdf/enews202329pdf.pdf (2023).
Yang, J. et al. Lenvatinib improves anti-PD-1 therapeutic efficacy by promoting vascular normalization via the NRP-1-PDGFRβ complex in hepatocellular carcinoma. Front. Immunol. 14, 1212577 (2023).
Tran, T. T. et al. Lenvatinib or anti-VEGF in combination with anti-PD-1 differentially augments antitumor activity in melanoma. JCI Insight 8, e157347 (2023).
Yamauchi, M. et al. Lenvatinib activates anti-tumor immunity by suppressing immunoinhibitory infiltrates in the tumor microenvironment of advanced hepatocellular carcinoma. Commun. Med. 3, 152 (2023).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Bocca, P. et al. Bevacizumab-mediated tumor vasculature remodelling improves tumor infiltration and antitumor efficacy of GD2-CAR T cells in a human neuroblastoma preclinical model. Oncoimmunology 7, e1378843 (2017).
Dong, X. et al. Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice. J. Immunother. Cancer 11, e005583 (2023).
Saharinen, P., Eklund, L. & Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 16, 635–661 (2017).
Park, H. R. et al. Angiopoietin-2-dependent spatial vascular destabilization promotes T-cell exclusion and limits immunotherapy in melanoma. Cancer Res. 83, 1968–1983 (2023).
Park, J. S. et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell 30, 953–967 (2016).
Kloepper, J. et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc. Natl Acad. Sci. USA 113, 4476–4481 (2016).
Schmittnaegel, M. et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 9, eaak9670 (2017).
Peterson, T. E. et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl Acad. Sci. USA 113, 4470–4475 (2016).
Hasegawa, Y. et al. Transforming growth factor-β1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 91, 964–971 (2001).
Thomas, D. A. & Massague, J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Zhang, F. et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 7, 52294–52306 (2016).
Polanczyk, M. J., Walker, E., Haley, D., Guerrouahen, B. S. & Akporiaye, E. T. Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4+CD25+Foxp3+ and CD4+CD25−Foxp3+ T cells. J. Transl. Med. 17, 219 (2019).
Liu, J. et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 109, 16618–16623 (2012).
Liao, S. et al. TGF-β blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin. Cancer Res. 17, 1415–1424 (2011).
Li, S. et al. Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature 587, 121–125 (2020).
Liu, M. et al. TGF-β suppresses type 2 immunity to cancer. Nature 587, 115–120 (2020).
Panagi, M. et al. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 10, 1910–1922 (2020).
Courau, T. et al. TGF-β and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight 1, e85974 (2016).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Horn, L. A. et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-β signaling enables PD-L1-mediated tumor eradication. J. Clin. Invest. 132, e155148 (2022).
Qin, Z. et al. A critical requirement of interferon γ-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 63, 4095–4100 (2003).
Strieter, R. M., Burdick, M. D., Gomperts, B. N., Belperio, J. A. & Keane, M. P. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 16, 593–609 (2005).
Sato, N. et al. Actions of TNF and IFN-γ on angiogenesis in vitro. J. Invest. Dermatol. 95, 85S–89S (1990).
Zheng, X. et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J. Clin. Invest. 128, 2104–2115 (2018).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Li, Q. et al. Low-dose anti-angiogenic therapy sensitizes breast cancer to PD-1 blockade. Clin. Cancer Res. 26, 1712–1724 (2020).
Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak9679 (2017).
Wu, F. T. H. et al. Efficacy of cotargeting angiopoietin-2 and the VEGF pathway in the adjuvant postsurgical setting for early breast, colorectal, and renal cancers. Cancer Res. 76, 6988–7000 (2016).
Di Tacchio, M. et al. Tumor vessel normalization, immunostimulatory reprogramming, and improved survival in glioblastoma with combined inhibition of PD-1, angiopoietin-2, and VEGF. Cancer Immunol. Res. 7, 1910–1927 (2019).
Yang, H. et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J. Clin. Invest. 129, 4350–4364 (2019).
Hodi, F. S. et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol. Res. 2, 632–642 (2014).
Wu, X. et al. VEGF neutralization plus CTLA-4 blockade alters soluble and cellular factors associated with enhancing lymphocyte infiltration and humoral recognition in melanoma. Cancer Immunol. Res. 4, 858–868 (2016).
Wallin, J. J. et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 7, 12624 (2016).
Liu, J. et al. Multicenter phase II trial of camrelizumab combined with apatinib and eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat. Commun. 13, 3011 (2022).
Quintela-Fandino, M. et al. Immuno-priming durvalumab with bevacizumab in HER2-negative advanced breast cancer: a pilot clinical trial. Breast Cancer Res. 22, 124 (2020).
Makker, V. et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 20, 711–718 (2019).
Li, X., Kostareli, E., Suffner, J., Garbi, N. & Hammerling, G. J. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur. J. Immunol. 40, 3325–3335 (2010).
Zheng, X. et al. CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Int. J. Cancer 146, 1730–1740 (2020).
Carretero, R. et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 16, 609–617 (2015).
Delyon, J. et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann. Oncol. 24, 1697–1703 (2013).
Griffiths, M. R., Botto, M., Morgan, B. P., Neal, J. W. & Gasque, P. CD93 regulates central nervous system inflammation in two mouse models of autoimmune encephalomyelitis. Immunology 155, 346–355 (2018).
Sun, Y. et al. Blockade of the CD93 pathway normalizes tumor vasculature to facilitate drug delivery and immunotherapy. Sci. Transl. Med. 13, eabc8922 (2021).
Palazon, A. et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 71, 801–811 (2011).
Hollenbaugh, D. et al. Expression of functional CD40 by vascular endothelial cells. J. Exp. Med. 182, 33–40 (1995).
Dechanet, J. et al. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells. J. Immunol. 159, 5640–5647 (1997).
Albini, A. et al. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: a gene therapy approach. Am. J. Pathol. 156, 1381–1393 (2000).
Chelvanambi, M., Fecek, R. J., Taylor, J. L. & Storkus, W. J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 9, e001906 (2021).
Lee, S. J. et al. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J. Immunother. Cancer 9, e002195 (2021).
Wang-Bishop, L. et al. STING-activating nanoparticles normalize the vascular-immune interface to potentiate cancer immunotherapy. Sci. Immunol. 8, eadd1153 (2023).
Lejeune, F. J., Lienard, D., Matter, M. & Ruegg, C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 6, 6 (2006).
Grunhagen, D. J., de Wilt, J. H., ten Hagen, T. L. & Eggermont, A. M. Technology insight: utility of TNF-α-based isolated limb perfusion to avoid amputation of irresectable tumors of the extremities. Nat. Clin. Pract. Oncol. 3, 94–103 (2006).
Curnis, F., Sacchi, A. & Corti, A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J. Clin. Invest. 110, 475–482 (2002).
Elia, A. R. et al. Targeting tumor vasculature with TNF leads effector T cells to the tumor and enhances therapeutic efficacy of immune checkpoint blockers in combination with adoptive cell therapy. Clin. Cancer Res. 24, 2171–2181 (2018).
Shen, J. et al. Vascular-targeted TNFα and IFNγ inhibits orthotopic colorectal tumor growth. J. Transl. Med. 14, 187 (2016).
Johansson-Percival, A. et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol. 18, 1207–1217 (2017).
Johansson-Percival, A. et al. Intratumoral LIGHT restores pericyte contractile properties and vessel integrity. Cell Rep. 13, 2687–2698 (2015).
He, B. et al. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J. Pathol. 245, 209–221 (2018).
Herrera, F. G. et al. Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 12, 108–133 (2022).
Herrera, F. G., Irving, M., Kandalaft, L. E. & Coukos, G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. 20, e417–e433 (2019).
Lugade, A. A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Cheng, J. N. et al. Radiation-induced eosinophils improve cytotoxic T lymphocyte recruitment and response to immunotherapy. Sci. Adv. 7, eabc7609 (2021).
Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013).
Mpekris, F., Baish, J. W., Stylianopoulos, T. & Jain, R. K. Role of vascular normalization in benefit from metronomic chemotherapy. Proc. Natl Acad. Sci. USA 114, 1994–1999 (2017).
Bocci, G. et al. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br. J. Cancer 98, 1619–1629 (2008).
Yousaf, I., Kaeppler, J., Frost, S., Seymour, L. W. & Jacobus, E. J. Attenuation of the hypoxia inducible factor pathway after oncolytic adenovirus infection coincides with decreased vessel perfusion. Cancers 12, 851 (2020).
Hou, W., Chen, H., Rojas, J., Sampath, P. & Thorne, S. H. Oncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGF. Int. J. Cancer 135, 1238–1246 (2014).
Frentzen, A. et al. Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumor therapy. Proc. Natl Acad. Sci. USA 106, 12915–12920 (2009).
Guse, K. et al. Antiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer models. J. Virol. 84, 856–866 (2010).
He, T. et al. Oncolytic adenovirus promotes vascular normalization and nonclassical tertiary lymphoid structure formation through STING-mediated DC activation. Oncoimmunology 11, 2093054 (2022).
Moon, E. K. et al. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology 7, e1395997 (2018).
Wang, G. et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol. Ther. 31, 134–153 (2023).
Garcia-Caballero, M., Sokol, L., Cuypers, A. & Carmeliet, P. Metabolic reprogramming in tumor endothelial cells. Int. J. Mol. Sci. 23, 11052 (2022).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Cantelmo, A. R. et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30, 968–985 (2016).
Shan, Y. et al. Targeting tumor endothelial hyperglycolysis enhances immunotherapy through remodeling tumor microenvironment. Acta Pharm. Sin. B. 12, 1825–1839 (2022).
Cui, J. et al. JP1 normalizes tumor vasculature to suppress metastasis and facilitate drug delivery by inhibiting IL-8. JCI Insight 8, e161675 (2023).
Zhang, D. et al. PHGDH-mediated endothelial metabolism drives glioblastoma resistance to chimeric antigen receptor T cell immunotherapy. Cell Metab. 35, 517–534.e8 (2023).
Andrea, A. E. et al. Advances in CAR-T cell genetic engineering strategies to overcome hurdles in solid tumors treatment. Front. Immunol. 13, 830292 (2022).
Watson, H. A. et al. L-selectin enhanced T cells improve the efficacy of cancer immunotherapy. Front. Immunol. 10, 1321 (2019).
Kochl, R. et al. Corrigendum: WNK1 kinase balances T cell adhesion versus migration in vivo. Nat. Immunol. 18, 246 (2017).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Yamamoto, T. N. et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J. Clin. Invest. 129, 1551–1565 (2019).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Chmielewski, M. & Abken, H. CAR T cells releasing IL-18 convert to T-Bethigh FoxO1low effectors that exhibit augmented activity against advanced solid tumors. Cell Rep. 21, 3205–3219 (2017).
Kerkar, S. P. et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest. 121, 4746–4757 (2011).
Kuhn, N. F. et al. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell 35, 473–488.e6 (2019).
Irving, M., Lanitis, E., Migliorini, D., Ivics, Z. & Guedan, S. Choosing the right tool for genetic engineering: clinical lessons from chimeric antigen receptor-T cells. Hum. Gene Ther. 32, 1044–1058 (2021).
Ciraolo, E. et al. Simultaneous genetic ablation of PD-1, LAG-3, and TIM-3 in CD8 T cells delays tumor growth and improves survival outcome. Int. J. Mol. Sci. 23, 3207 (2022).
Wang, W. et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency. Gene Ther. 20, 970–978 (2013).
Chinnasamy, D. et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J. Clin. Invest. 120, 3953–3968 (2010).
Ash, S. L. et al. Targeting the activated microenvironment with endosialin (CD248)-directed CAR-T cells ablates perivascular cells to impair tumor growth and metastasis. J. Immunother. Cancer 12, e008608 (2024).
Fierle, J. K. et al. Soluble trivalent engagers redirect cytolytic T cell activity toward tumor endothelial marker 1. Cell Rep. Med. 2, 100362 (2021).
Xie, Y. J. et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl Acad. Sci. USA 116, 7624–7631 (2019).
Kershaw, M. H. et al. Generation of gene-modified T cells reactive against the angiogenic kinase insert ___domain-containing receptor (KDR) found on tumor vasculature. Hum. Gene Ther. 11, 2445–2452 (2000).
Xing, H. et al. Anti-tumor effects of vascular endothelial growth factor/vascular endothelial growth factor receptor binding ___domain-modified chimeric antigen receptor T cells. Cytotherapy 23, 810–819 (2021).
Lanitis, E. et al. VEGFR-2 redirected CAR-T cells are functionally impaired by soluble VEGF-A competition for receptor binding. J. Immunother. Cancer 9, e002151 (2021).
Lanitis, E. et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression. J. Exp. Med. 218, e20192203 (2021).
Byrd, T. T. et al. TEM8/ANTXR1-specific CAR T cells as a targeted therapy for triple-negative breast cancer. Cancer Res. 78, 489–500 (2018).
Petrovic, K. et al. TEM8/ANTXR1-specific CAR T cells mediate toxicity in vivo. PLoS ONE 14, e0224015 (2019).
Santoro, S. P. et al. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol. Res. 3, 68–84 (2015).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Zhuang, X. et al. CAR T cells targeting tumor endothelial marker CLEC14A inhibit tumor growth. JCI Insight 5, e138808 (2020).
Lanitis, E., Coukos, G. & Irving, M. All systems go: converging synthetic biology and combinatorial treatment for CAR-T cell therapy. Curr. Opin. Biotechnol. 65, 75–87 (2020).
Chinnasamy, D. et al. Simultaneous targeting of tumor antigens and the tumor vasculature using T lymphocyte transfer synergize to induce regression of established tumors in mice. Cancer Res. 73, 3371–3380 (2013).
Lee, H. et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 23, 4190–4202 (2017).
Garaud, S., Dieu-Nosjean, M. C. & Willard-Gallo, K. T follicular helper and B cell crosstalk in tertiary lymphoid structures and cancer immunotherapy. Nat. Commun. 13, 2259 (2022).
Skandha Gopalan, K. & Bergers, G. The pan-tumor vasculature under the transcriptomic magnifying glass. Cancer Res. 84, 3502–3504 (2024).
Onder, L. et al. Fibroblastic reticular cells generate protective intratumoral T cell environments in lung cancer. Cell 188, 430–446.e20 (2024).
Greene, E. et al. New interpretable machine-learning method for single-cell data reveals correlates of clinical response to cancer immunotherapy. Patterns 2, 100372 (2021).
Acknowledgements
The authors thank the Ludwig Institute for Cancer Research, the University of Lausanne and the Lausanne University Hospital (CHUV), the Swiss National Science Foundation (SNSF# 310030_204326), the ISREC Foundation, the Fondazione Teofilo Rossi di Montelera e di Premuda, Cancera Foundation, and Biltema Foundation for their generous research support over the years. For this work, G.C. was funded by the Ludwig Institute for Cancer Research, as well as the CHUV and UNIL, and by generous grants from the Swiss National Science Foundation, Oncosuisse, the ISREC Foundation, Cancera Foundation, Mats Paulsson Foundation, and Biltema Foundation.
Author information
Authors and Affiliations
Contributions
The authors contributed to all aspects of the article. E.L. and M.I. contributed equally.
Corresponding author
Ethics declarations
Competing interests
G.C. has received honoraria from Bristol-Myers Squibb. The CHUV has received honoraria for advisory services provided by G.C. to Iovance and EVIR. G.C. has also has received royalties from the University of Pennsylvania for CAR T cell therapy licensed to Novartis and Tmunity Therapeutics, and royalties from the Ludwig Institute for Cancer Research, UNIL and CHUV for NeoTIL intellectual property previously licensed to Tigen Pharma. G.C. is inventor in technologies related to T cell expansion and engineering for T cell therapy. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Yi Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lanitis, E., Irving, M. & Coukos, G. Tumour-associated vasculature in T cell homing and immunity: opportunities for cancer therapy. Nat Rev Immunol (2025). https://doi.org/10.1038/s41577-025-01187-w
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-025-01187-w