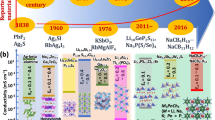
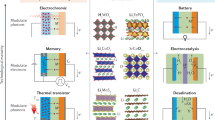
Abstract
Developing high-performance rechargeable batteries requires a revolutionary advancement in battery materials, guided by a fundamental understanding of their underlying science and mechanisms. However, this task remains a challenge owing to the complex relationship among composition, structure and property in electrode and electrolyte materials. Ionic potential, a concept derived from geochemistry, has been incorporated into battery materials research since 2020 as a methodology for predicting and optimizing their functional properties. Defined as the ratio of charge number of an ion to its ionic radius, ionic potential serves as a measure of the interaction strength within the structure of a material. In this Perspective, we explore the role of ionic potential in guiding the design of advanced materials for rechargeable batteries. Specifically, we discuss how integrating ionic potential into material design frameworks can capture critical structural interactions, thereby enabling improvements in properties such as ionic conductivity, redox activity and phase transition behaviours. Furthermore, we identify the broader relevance of ionic potential in battery systems, highlighting its potential in advancing fundamental understanding and performance capabilities in battery technology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Whittingham, M. S. Electrical energy storage and intercalation chemistry. Science 192, 1126–1127 (1976).
Goodenough, J. B. Evolution of strategies for modern rechargeable batteries. Acc. Chem. Res. 46, 1053–1061 (2013).
Godshall, N. A., Raistrick, I. D. & Huggins, R. A. Thermodynamic investigations of ternary lithium-transition metal-oxygen cathode materials. Mater. Res. Bull. 15, 561–570 (1980).
Mizushima, K., Jones, P. C., Wiseman, P. J. & Goodenough, J. B. LixCoO2 (0<x<1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980).
Ozawa, K. Lithium-ion rechargeable batteries with LiCoO2 and carbon electrodes: the LiCoO2/C system. Solid State Ion. 69, 212–221 (1994).
Reimers, J. N. & Dahn, J. R. Electrochemical and in situ X‐ray diffraction studies of lithium intercalation in LixCoO2. J. Electrochem. Soc. 139, 2091–2097 (1992).
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014).
Kim, S.-W., Seo, D.-H., Ma, X., Ceder, G. & Kang, K. Electrode materials for rechargeable sodium-ion batteries: potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2, 710–721 (2012).
Slater, M. D., Kim, D., Lee, E. & Johnson, C. S. Sodium-ion batteries. Adv. Funct. Mater. 23, 947–958 (2013).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Gong, Z. & Yang, Y. Recent advances in the research of polyanion-type cathode materials for Li-ion batteries. Energy Environ. Sci. 4, 3223–3242 (2011).
Kim, T., Song, W., Son, D.-Y., Ono, L. K. & Qi, Y. Lithium-ion batteries: outlook on present, future, and hybridized technologies. J. Mater. Chem. A 7, 2942–2964 (2019).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Chen, R., Li, Q., Yu, X., Chen, L. & Li, H. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem. Rev. 120, 6820–6877 (2020).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Pan, H., Hu, Y.-S. & Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 6, 2338–2360 (2013).
Oses, C., Toher, C. & Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 5, 295–309 (2020).
Schweidler, S. et al. High-entropy materials for energy and electronic applications. Nat. Rev. Mater. 9, 266–281 (2024).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976).
Cartledge, G. H. Studies on the periodic system. i. The ionic potential as a periodic function1. J. Am. Chem. Soc. 50, 2855–2863 (1928).
Railsback, L. B. An earth scientist’s periodic table of the elements and their ions. Geology 31, 737–740 (2003).
Thackeray, M. M., David, W. I. F., Bruce, P. G. & Goodenough, J. B. Lithium insertion into manganese spinels. Mater. Res. Bull. 18, 461–472 (1983).
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho‐olivines as positive‐electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188 (1997).
Hyooma, H. & Hayashi, K. Crystal structures of La3Li5M2O12 (M = Nb, Ta). Mater. Res. Bull. 23, 1399–1407 (1988).
Deiseroth, H.-J. et al. Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew. Chem. Int. Ed. 47, 755–758 (2008).
Railsback, L. B. An earth scientist’s periodic table of the elements and their ions. GSA Bull. 117, 746–746 (2005).
Railsback, L. B. Some fundamentals of mineralogy and geochemistry. Univ. Georgia https://railsback.org/FundamentalsIndex.html (2006).
Sun, Y., Guo, S. & Zhou, H. Adverse effects of interlayer-gliding in layered transition-metal oxides on electrochemical sodium-ion storage. Energy Environ. Sci. 12, 825–840 (2019).
Rong, X. et al. Anionic redox reaction-induced high-capacity and low-strain cathode with suppressed phase transition. Joule 3, 503–517 (2019).
Yu, H. et al. An ultrastable anode for long-life room-temperature sodium-ion batteries. Angew. Chem. Int. Ed. 53, 8963–8969 (2014).
Thackeray, M. M. et al. Spinel electrodes for lithium batteries — a review. J. Power Sources 21, 1–8 (1987).
Kim, H. et al. Understanding the electrochemical mechanism of the new iron-based mixed-phosphate Na4Fe3(PO4)2(P2O7) in a Na rechargeable battery. Chem. Mater. 25, 3614–3622 (2013).
Lee, B. et al. First-principles study of the reaction mechanism in sodium–oxygen batteries. Chem. Mater. 26, 1048–1055 (2014).
Jian, Z. et al. Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 cathode for room-temperature sodium-ion batteries. Adv. Energy Mater. 3, 156–160 (2013).
Kawai, K., Zhao, W., Nishimura, S. & Yamada, A. High-voltage Cr4+/Cr3+ redox couple in polyanion compounds. ACS Appl. Energy Mater. 1, 928–931 (2018).
Jiang, Y. et al. Nanoconfined carbon-coated Na3V2(PO4)3 particles in mesoporous carbon enabling ultralong cycle life for sodium-ion batteries. Adv. Energy Mater. 5, 1402104 (2015).
Zhao, C. et al. Novel methods for sodium-ion battery materials. Small Methods 1, 1600063 (2017).
Kim, H. et al. Recent progress in electrode materials for sodium-ion batteries. Adv. Energy Mater. 6, 1600943 (2016).
Yabuuchi, N. et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 11, 512 (2012).
Delmas, C., Fouassier, C. & Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C 99, 81–85 (1980).
Fouassier, C., Delmas, C. & Hagenmuller, P. Evolution structurale et proprietes physiques des phases AxMO2 (A = Na, K; M = Cr, Mn, Co) (x ≤ 1). Mater. Res. Bull. 10, 443–449 (1975).
Komaba, S. et al. Study on the reversible electrode reaction of Na1−xNi0.5Mn0.5O2 for a rechargeable sodium-ion battery. Inorg. Chem. 51, 6211–6220 (2012).
Lu, Z. & Dahn, J. R. In situ X-ray diffraction study of P2Na2/3[Ni1/3Mn2/3]O2. J. Electrochem. Soc. 148, A1225–A1229 (2001).
Billaud, J. et al. Na0.67Mn1−xMgxO2 (0≤x≤0.2): a high capacity cathode for sodium-ion batteries. Energy Environ. Sci. 7, 1387–1391 (2014).
Gupta, A., Buddie Mullins, C. & Goodenough, J. B. Na2Ni2TeO6: evaluation as a cathode for sodium battery. J. Power Sources 243, 817–821 (2013).
Li, Z.-Y. et al. New insights into designing high-rate performance cathode materials for sodium ion batteries by enlarging the slab-spacing of the Na-ion diffusion layer. J. Mater. Chem. A 4, 3453–3461 (2016).
Su, J., Pei, Y., Yang, Z. & Wang, X. First-principles investigation on the structural, electronic properties and diffusion barriers of Mg/Al doped NaCoO2 as the cathode material of rechargeable sodium batteries. RSC Adv. 5, 27229–27234 (2015).
Han, S. C. et al. Ca-doped NaxCoO2 for improved cyclability in sodium ion batteries. J. Power Sources 277, 9–16 (2015).
Sathiya, M. et al. A chemical approach to raise cell voltage and suppress phase transition in O3 sodium layered oxide electrodes. Adv. Energy Mater. 8, 1702599 (2018).
Aguesse, F. et al. Structural and electrochemical analysis of Zn doped Na3Ni2SbO6 cathode for Na-ion battery. J. Power Sources 336, 186–195 (2016).
Yuan, D. et al. A honeycomb-layered Na3Ni2SbO6: a high-rate and cycle-stable cathode for sodium-ion batteries. Adv. Mater. 26, 6301–6306 (2014).
Seibel, E. M., Roudebush, J. H., Ali, M. N., Ross, K. A. & Cava, R. J. Structure and magnetic properties of the spin-1/2-based honeycomb NaNi2BiO6−δ and its hydrate NaNi2BiO6−δ·1.7H2O. Inorg. Chem. 53, 10989–10995 (2014).
Bhange, D. S. et al. Honeycomb-layer structured Na3Ni2BiO6 as a high voltage and long life cathode material for sodium-ion batteries. J. Mater. Chem. A 5, 1300–1310 (2017).
Claude D., Fouassier, C. & Hagenmuller, P. Stabilite relative des environnements octaedrique et prismatique triangulaire dans les oxydes lamellaires alcalins AxMO2 (x≤1). Mater. Res. Bull. 11, 1483–1488 (1976).
Rouxel, J. Sur un diagramme ionicité-structure pour les composes intercalaires alcalins des sulfures lamellaires. J. Solid State Chem. 17, 223–229 (1976).
Pauling, L. The Nature of the Chemical Bond 3rd edn (Cornell Univ. Press, 1960).
Guilmard, M., Croguennec, L. & Delmas, C. Effects of manganese substitution for nickel on the structural and electrochemical properties of LiNiO2. J. Electrochem. Soc. 150, A1287–A1293 (2003).
Zhao, C., Avdeev, M., Chen, L. & Hu, Y.-S. An O3-type oxide with low sodium content as the phase-transition-free anode for sodium-ion batteries. Angew. Chem. Int. Ed. 57, 7056–7060 (2018).
Zhao, C. et al. Rational design of layered oxide materials for sodium-ion batteries. Science 370, 708–711 (2020).
Shin, Y.-J. & Yi, M.-Y. Preparation and structural properties of layer-type oxides NaxNix/2Ti1−x/2O2 (0.60≤x≤1.0). Solid State Ion. 132, 131–141 (2000).
Singh, G. et al. High voltage Mg-doped Na0.67Ni0.3−xMgxMn0.7O2 (x=0.05, 0.1) Na-ion cathodes with enhanced stability and rate capability. Chem. Mater. 28, 5087–5094 (2016).
Wang, Q. et al. Unlocking anionic redox activity in O3-type sodium 3d layered oxides via Li substitution. Nat. Mater. 20, 353–361 (2021).
Sato, T., Sato, K., Zhao, W., Kajiya, Y. & Yabuuchi, N. Metastable and nanosize cation-disordered rocksalt-type oxides: revisit of stoichiometric LiMnO2 and NaMnO2. J. Mater. Chem. A 6, 13943–13951 (2018).
Uyama, T., Mukai, K. & Yamada, I. Synthesis of rhombohedral LiCo0.64Mn0.36O2 using a high-pressure method. Inorg. Chem. 58, 6684–6695 (2019).
Han, M. H. et al. Synthesis and electrochemistry study of P2- and O3-phase Na2/3Fe1/2Mn1/2O2. Electrochim. Acta 182, 1029–1036 (2015).
Guo, H. et al. Predominant P3-type solid–solution phase transition enables high-stability O3-type Na-ion cathodes. ACS Appl. Mater. Interfaces 16, 27352–27359 (2024).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46, 7778–7781 (2007).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Sakuda, A., Hayashi, A., Ohtomo, T., Hama, S. & Tatsumisago, M. All-solid-state lithium secondary batteries using LiCoO2 particles with pulsed laser deposition coatings of Li2S–P2S5 solid electrolytes. J. Power Sources 196, 6735–6741 (2011).
Maekawa, H. et al. Halide-stabilized LiBH4, a room-temperature lithium fast-ion conductor. J. Am. Chem. Soc. 131, 894–895 (2009).
Asano, T. et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 30, 1803075 (2018).
Li, X. et al. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 13, 1429–1461 (2020).
Kochetkov, I. et al. Different interfacial reactivity of lithium metal chloride electrolytes with high voltage cathodes determines solid-state battery performance. Energy Environ. Sci. 15, 3933–3944 (2022).
Kwak, H. et al. Boosting the interfacial superionic conduction of halide solid electrolytes for all-solid-state batteries. Nat. Commun. 14, 2459 (2023).
Hu, L. et al. A cost-effective, ionically conductive and compressible oxychloride solid-state electrolyte for stable all-solid-state lithium-based batteries. Nat. Commun. 14, 3807 (2023).
Ishiguro, Y., Ueno, K., Nishimura, S., Iida, G. & Igarashib, Y. TaCl5-glassified ultrafast lithium ion-conductive halide electrolytes for high-performance all-solid-state lithium batteries. Chem. Lett. 52, 237–241 (2023).
Wang, S. et al. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability. Angew. Chem. Int. Ed. 58, 8039–8043 (2019).
Wang, C., Liang, J., Kim, J. T. & Sun, X. Prospects of halide-based all-solid-state batteries: from material design to practical application. Sci. Adv. 8, eadc9516 (2022).
Zhou, L. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022).
Louli, A. J. et al. Exploring the impact of mechanical pressure on the performance of anode-free lithium metal cells. J. Electrochem. Soc. 166, A1291 (2019).
Schlem, R. et al. Mechanochemical synthesis: a tool to tune cation site disorder and ionic transport properties of Li3MCl6 (M=Y, Er) superionic conductors. Adv. Energy Mater. 10, 1903719 (2020).
Kwak, H. et al. Li+ conduction in aliovalent-substituted monoclinic Li2ZrCl6 for all-solid-state batteries: Li2+xZr1−xMxCl6 (M=In, Sc). Chem. Eng. J. 437, 135413 (2022).
Mouta, R., Melo, M. Á. B., Diniz, E. M. & Paschoal, C. W. A. Concentration of charge carriers, migration, and stability in Li3OCl solid electrolytes. Chem. Mater. 26, 7137–7144 (2014).
Zhao, C. et al. Solid-state sodium batteries. Adv. Energy Mater. 8, 1703012 (2018).
Jun, K., Chen, Y., Wei, G., Yang, X. & Ceder, G. Diffusion mechanisms of fast lithium-ion conductors. Nat. Rev. Mater. 9, 887–905 (2024).
Liu, Z. et al. Tuning collective anion motion enables superionic conductivity in solid-state halide electrolytes. Nat. Chem. 16, 1584–1591 (2024).
Bohnsack, A. et al. Ternäre halogenide vom Typ A3MX6. VI [1]. Ternäre Chloride der Selten-Erd-Elemente mit lithium, Li3MCl6 (M=Tb-Lu, Y, Sc): synthese, kristallstrukturen und ionenbewegung. Z. Anorg. Allg. Chem. 623, 1067–1073 (1997).
Wang, K. et al. A cost-effective and humidity-tolerant chloride solid electrolyte for lithium batteries. Nat. Commun. 12, 4410 (2021).
Elliott, S. R. in Physics of Amorphous Materials 2nd edn 139–151 (Longman, 1990).
Greer, A. L. Intermetallic Compounds — Principles and Practice Vol. 1 (eds Westbrook, J. H. & Fleischer, R. L.) 731–754 (Wiley, 1995).
Sheng, H. W., Luo, W. K., Alamgir, F. M., Bai, J. M. & Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 439, 419–425 (2006).
Zhang, N. et al. The missing boundary in the phase diagram of PbZr1−xTixO3. Nat. Commun. 5, 5231 (2014).
Yang, T. et al. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys. Science 362, 933–937 (2018).
Zhang, R. et al. Short-range order and its impact on the CrCoNi medium-entropy alloy. Nature 581, 283–287 (2020).
Wang, Q. et al. Designing lithium halide solid electrolytes. Nat. Commun. 15, 1050 (2024).
Antaya, M., Cearns, K., Preston, J. S., Reimers, J. N. & Dahn, J. R. In situ growth of layered, spinel, and rock‐salt LiCoO2 by laser ablation deposition. J. Appl. Phys. 76, 2799–2806 (1994).
Kanno, R. et al. Synthesis, structure, and electrochemical properties of a new lithium iron oxide, LiFeO2, with a corrugated layer structure. J. Electrochem. Soc. 143, 2435 (1996).
Sakurai, Y., Arai, H. & Yamaki, J.-i Preparation of electrochemically active α-LiFeO2 at low temperature. Solid State Ion. 113–115, 29–34 (1998).
Werder, D. J., Chen, C. H., Cava, R. J. & Batlogg, B. Diffraction evidence for oxygen-vacancy ordering in annealed Ba2YCu3O7 (0.3≤δ≤0.4) superconductors. Phys. Rev. B 37, 2317–2319 (1988).
Li, L. et al. Evolution of short-range order and its effects on the plastic deformation behavior of single crystals of the equiatomic Cr-Co-Ni medium-entropy alloy. Acta Mater. 243, 118537 (2023).
Rossen, E., Reimers, J. N. & Dahn, J. R. Synthesis and electrochemistry of spinel LT-LiCoO2. Solid State Ion. 62, 53–60 (1993).
Gummow, R. J., Thackeray, M. M., David, W. I. F. & Hull, S. Structure and electrochemistry of lithium cobalt oxide synthesised at 400 °C. Mater. Res. Bull. 27, 327–337 (1992).
Wang, Q. et al. Chemical short-range disorder in lithium oxide cathodes. Nature 629, 341–347 (2024).
Ong, S. P. et al. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 4, 3680–3688 (2011).
Okoshi, M., Yamada, Y., Yamada, A. & Nakai, H. Theoretical analysis on de-solvation of lithium, sodium, and magnesium cations to organic electrolyte solvents. J. Electrochem. Soc. 160, A2160–A2165 (2013).
Han, M. H., Gonzalo, E., Singh, G. & Rojo, T. A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries. Energy Environ. Sci. 8, 81–102 (2015).
Zhao, C., Ding, F., Lu, Y., Chen, L. & Hu, Y.-S. High-entropy layered oxide cathodes for sodium-ion batteries. Angew. Chem. Int. Ed. 59, 264–269 (2020).
Kubota, K., Kumakura, S., Yoda, Y., Kuroki, K. & Komaba, S. Electrochemistry and solid-state chemistry of NaMeO2 (Me=3d transition metals). Adv. Energy Mater. 8, 1703415 (2018).
Zhao, C. et al. Ti substitution facilitating oxygen oxidation in Na2/3Mg1/3Ti1/6Mn1/2O2 cathode. Chem 5, 2913–2925 (2019).
Kubota, K. et al. Impact of Mg and Ti doping in O3 type NaNi1/2Mn1/2O2 on reversibility and phase transition during electrochemical Na intercalation. J. Mater. Chem. A 9, 12830–12844 (2021).
Han, M. H., Gonzalo, E., Casas-Cabanas, M. & Rojo, T. Structural evolution and electrochemistry of monoclinic NaNiO2 upon the first cycling process. J. Power Sources 258, 266–271 (2014).
Zhou, Y.-N. et al. Phase transition behavior of NaCrO2 during sodium extraction studied by synchrotron-based X-ray diffraction and absorption spectroscopy. J. Mater. Chem. A 1, 11130–11134 (2013).
Delmas, C., Braconnier, J.-J., Fouassier, C. & Hagenmuller, P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ion. 3–4, 165–169 (1981).
Xie, Y. et al. In operando XRD and TXM study on the metastable structure change of NaNi1/3Fe1/3Mn1/3O2 under electrochemical sodium-ion intercalation. Adv. Energy Mater. 6, 1601306 (2016).
Lee, E. et al. New insights into the performance degradation of Fe-based layered oxides in sodium-ion batteries: instability of Fe3+/Fe4+ redox in α-NaFeO2. Chem. Mater. 27, 6755–6764 (2015).
Susanto, D. et al. Anionic redox activity as a key factor in the performance degradation of NaFeO2 cathodes for sodium ion batteries. Chem. Mater. 31, 3644–3651 (2019).
Vassilaras, P. et al. Electrochemical properties and structural evolution of O3-type layered sodium mixed transition metal oxides with trivalent nickel. J. Mater. Chem. A 5, 4596–4606 (2017).
Ding, F. et al. A novel Ni-rich O3-Na[Ni0.60Fe0.25Mn0.15]O2 cathode for Na-ion batteries. Energy Storage Mater. 30, 420–430 (2020).
Wang, Q. et al. Reaching the energy density limit of layered O3-NaNi0.5Mn0.5O2 electrodes via dual Cu and Ti substitution. Adv. Energy Mater. 9, 1901785 (2019).
Ding, F. et al. Using high-entropy configuration strategy to design Na-ion layered oxide cathodes with superior electrochemical performance and thermal stability. J. Am. Chem. Soc. 144, 8286–8295 (2022).
Maletti, S., Sarapulova, A., Schökel, A. & Mikhailova, D. Operando studies on the NaNi0.5Ti0.5O2 cathode for Na-ion batteries: elucidating titanium as a structure stabilizer. ACS Appl. Mater. Interfaces 11, 33923–33930 (2019).
Wang, P.-F. et al. An abnormal 3.7-volt O3-type sodium-ion battery cathode. Angew. Chem. Int. Ed. 57, 8178–8183 (2018).
Wang, Q. et al. Fast-charge high-voltage layered cathodes for sodium-ion batteries. Nat. Sustain. 7, 338–347 (2024).
Liu, C., Neale, Z. G. & Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 19, 109–123 (2016).
Wu, D. et al. NaTiO2: a layered anode material for sodium-ion batteries. Energy Environ. Sci. 8, 195–202 (2015).
Billaud, J. et al. β-NaMnO2: a high-performance cathode for sodium-ion batteries. J. Am. Chem. Soc. 136, 17243–17248 (2014).
Ma, X., Chen, H. & Ceder, G. Electrochemical properties of monoclinic NaMnO2. J. Electrochem. Soc. 158, A1307–A1312 (2011).
Jo, I.-H. et al. The effect of electrolyte on the electrochemical properties of Na/α-NaMnO2 batteries. Mater. Res. Bull. 58, 74–77 (2014).
Clément, R. J., Middlemiss, D. S., Seymour, I. D., Ilott, A. J. & Grey, C. P. Insights into the nature and evolution upon electrochemical cycling of planar defects in the β-NaMnO2 Na-ion battery cathode: an NMR and first-principles density functional theory approach. Chem. Mater. 28, 8228–8239 (2016).
Cabana, J. et al. Study of the transition metal ordering in layered NaxNix/2Mn1−x/2O2 (2/3≤x≤1) and consequences of Na/Li exchange. Inorg. Chem. 52, 8540–8550 (2013).
Vassilaras, P., Ma, X., Li, X. & Ceder, G. Electrochemical properties of monoclinic NaNiO2. J. Electrochem. Soc. 160, A207 (2013).
Zhao, C., Lu, Y., Chen, L. & Hu, Y.-S. Ni-based cathode materials for Na-ion batteries. Nano Res. 12, 2018–2030 (2019).
Zhou, P. et al. Synthesis, structure, and electrochemical properties of O′3-type monoclinic NaNi0.8Co0.15Al0.05O2 cathode materials for sodium-ion batteries. J. Mater. Chem. 7, 657–663 (2019).
Vassilaras, P., Toumar, A. J. & Ceder, G. Electrochemical properties of NaNi1/3Co1/3Fe1/3O2 as a cathode material for Na-ion batteries. Electrochem. Commun. 38, 79–81 (2014).
Yuan, D. D., Wang, Y. X., Cao, Y. L., Ai, X. P. & Yang, H. X. Improved electrochemical performance of Fe-substituted NaNi0.5Mn0.5O2 cathode materials for sodium-ion batteries. ACS Appl. Mater. Interfaces 7, 8585–8591 (2015).
Alvarado, J. et al. Improvement of the cathode electrolyte interphase on P2-Na2/3Ni1/3Mn2/3O2 by atomic layer deposition. ACS Appl. Mater. Interfaces 9, 26518–26530 (2017).
Shanmugam, R. & Lai, W. Na2/3Ni1/3Ti2/3O2: ‘bi-functional’ electrode materials for Na-ion batteries. ECS Electrochem. Lett. 3, A23–A25 (2014).
Kurbakov, A. I. et al. Long-range and short-range ordering in 2D honeycomb-lattice magnet Na2Ni2TeO6. J. Alloy Compd. 820, 153354 (2020).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Thackeray, M. M., David, W. I. F. & Goodenough, J. B. Structural characterization of the lithiated iron oxides LixFe3O4 and LixFe2O3 (0<x<2). Mater. Res. Bull. 17, 785–793 (1982).
Masquelier, C. & Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 113, 6552–6591 (2013).
Jian, Z. et al. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem. Commun. 14, 86–89 (2012).
Barpanda, P., Oyama, G., Nishimura, S.-I, Chung, S.-C. & Yamada, A. A 3.8-V earth-abundant sodium battery electrode. Nat. Commun. 5, 4358 (2014).
Morgan, B. J. Mechanistic origin of superionic lithium diffusion in anion-disordered Li6PS5X argyrodites. Chem. Mater. 33, 2004–2018 (2021).
Kraft, M. A. et al. Influence of lattice polarizability on the ionic conductivity in the lithium superionic argyrodites Li6PS5X (X=Cl, Br, I). J. Am. Chem. Soc. 139, 10909–10918 (2017).
Stamminger, A. R., Ziebarth, B., Mrovec, M., Hammerschmidt, T. & Drautz, R. Ionic conductivity and its dependence on structural disorder in halogenated argyrodites Li6PS5X (X = Br, Cl, I). Chem. Mater. 31, 8673–8678 (2019).
Hartel, J. et al. Understanding lithium-ion transport in selenophosphate-based lithium argyrodites and their limitations in solid-state batteries. Chem. Mater. 35, 4798–4809 (2023).
Schwietert, T. K. et al. Understanding the role of aliovalent cation substitution on the Li-ion diffusion mechanism in Li6+xP1−xSixS5Br argyrodites. Mater. Adv. 5, 1952–1959 (2024).
Bernges, T., Culver, S. P., Minafra, N., Koerver, R. & Zeier, W. G. Competing structural influences in the Li superionic conducting argyrodites Li6PS5−xSexBr (0≤x≤1) upon Se substitution. Inorg. Chem. 57, 13920–13928 (2018).
Lavrinenko, A. K. et al. Optimizing ionic transport in argyrodites: a unified view on the role of sulfur/halide distribution and local environments. J. Mater. Chem. A 12, 26596–26611 (2024).
Lee, J. et al. Disorder-dependent Li diffusion in Li6PS5Cl investigated by machine-learning potential. ACS Appl. Mater. Interfaces 16, 46442–46453 (2024).
Wang, P. et al. Fast ion conduction and its origin in Li6−xPS5−xBr1+x. Chem. Mater. 32, 3833–3840 (2020).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Feng, X., Chien, P.-H., Patel, S., Wang, Y. & Hu, Y.-Y. Enhanced ion conduction in Li2.5Zn0.25PS4 via anion doping. Chem. Mater. 32, 3036–3042 (2020).
You, Y., Celio, H., Li, J., Dolocan, A. & Manthiram, A. Modified high-nickel cathodes with stable surface chemistry against ambient air for lithium-ion batteries. Angew. Chem. Int. Ed. 57, 6480–6485 (2018).
Shizuka, K., Kiyohara, C., Shima, K. & Takeda, Y. Effect of CO2 on layered Li1+zNi1−x−yCoxMyO2 (M=Al, Mn) cathode materials for lithium ion batteries. J. Power Sources 166, 233–238 (2007).
Sun, Y. et al. Degradation mechanism of O3-type NaNi1/3Fe1/3Mn1/3O2 cathode materials during ambient storage and their in situ regeneration. ACS Appl. Energy Mater. 4, 2061–2067 (2021).
Li, H. et al. Universal design strategy for air-stable layered Na-ion cathodes toward sustainable energy storage. Adv. Mater. 36, 2403073 (2024).
Liu, D. et al. Recent progress in sulfide-based solid electrolytes for Li-ion batteries. Mater. Sci. Eng. B 213, 169–176 (2016).
Tsukasaki, H. et al. Deterioration process of argyrodite solid electrolytes during exposure to humidity-controlled air. J. Power Sources 524, 231085 (2022).
Chen, X. et al. Improved stability against moisture and lithium metal by doping F into Li3InCl6. J. Power Sources 545, 231939 (2022).
Li, X. et al. Water-mediated synthesis of a superionic halide solid electrolyte. Angew. Chem. Int. Ed. 58, 16427–16432 (2019).
Zhan, C., Wu, T., Lu, J. & Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes — a critical review. Energy Environ. Sci. 11, 243–257 (2018).
Choi, W. & Manthiram, A. Comparison of metal ion dissolutions from lithium ion battery cathodes. J. Electrochem. Soc. 153, A1760 (2006).
Yang, Y. et al. Decoupling the air sensitivity of Na-layered oxides. Science 385, 744–752 (2024).
Langmuir, D. Aqueous environmental geochemistry. Eos Trans. AGU 78, 586–586 (1997).
Dzombak, D. A. & Morel, F. M. M. Surface Complexation Modeling: Hydrous Ferric Oxide (Wiley, 1991).
Yao, L.-H., Cao, W.-Q., Shu, J.-C., Cao, M.-S. & Sun, X.-D. Tailoring adsorption for tunable lithium ion storage and devices. Chem. Eng. J. 413, 127428 (2021).
Zhang, M. et al. Adsorption-catalysis design in the lithium–sulfur battery. Adv. Energy Mater. 10, 1903008 (2020).
Yan, C. et al. Toward critical electrode/electrolyte interfaces in rechargeable batteries. Adv. Funct. Mater. 30, 1909887 (2020).
Zhang, J.-N. et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy 4, 594–603 (2019).
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Pan, H. et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 1, 16039 (2016).
Wang, Q. et al. High entropy liquid electrolytes for lithium batteries. Nat. Commun. 14, 440 (2023).
Wang, Q. et al. Entropy-driven liquid electrolytes for lithium batteries. Adv. Mater. 35, 2210677 (2023).
Wang, Q. et al. Clarifying the relationship between the lithium deposition coverage and microstructure in lithium metal batteries. J. Am. Chem. Soc. 144, 21961–21971 (2022).
Wang, Q. et al. Interphase design for lithium-metal anodes. J. Am. Chem. Soc. 147, 9365–9377 (2025).
Bowen, N. L. The reaction principle in petrogenesis. J. Geol. 30, 177–198 (1922).
Acknowledgements
The authors extend sincere gratitude to L. B. Railsback from the University of Georgia for granting permission to adapt his original figures in this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the discussion and researched data for the manuscript. Q.W. and C.Z. prepared and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Hu, YS., Li, H. et al. Ionic potential for battery materials. Nat Rev Mater (2025). https://doi.org/10.1038/s41578-025-00822-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41578-025-00822-1