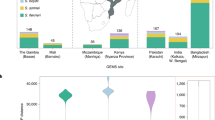
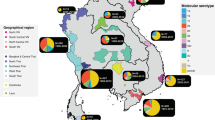
Abstract
Shigella sonnei is a major cause of diarrhoea globally and is increasing in prevalence relative to other Shigella because of multiple demographic and environmental influences. This single-serotype species has traditionally received less attention in comparison to Shigella flexneri and Shigella dysenteriae, which were more common in low-income countries and more tractable in the laboratory. In recent years, we have learned that Shigella are highly complex and highly susceptible to environmental change, as exemplified by epidemiological trends and increasing relevance of S. sonnei. Ultimately, methods, tools and data generated from decades of detailed research into S. flexneri have been used to gain new insights into the epidemiology, microbiology and pathogenesis of S. sonnei. In parallel, widespread adoption of genomic surveillance has yielded insights into antimicrobial resistance, evolution and organism transmission. In this Review, we provide an overview of current knowledge of S. sonnei, highlighting recent insights into this globally disseminated antimicrobial-resistant pathogen and assessing how novel data may impact future vaccine development and implementation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kotloff, K. L., Riddle, M. S., Platts-Mills, J. A., Pavlinac, P. & Zaidi, A. K. M. Shigellosis. Lancet 391, 801–812 (2018).
Anderson, J. D. et al. Burden of enterotoxigenic Escherichia coli and Shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob. Health 7, e321–e330 (2019).
Khalil, I. A. et al. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 18, 1229–1240 (2018).
Baker, K. S. et al. Travel- and community-based transmission of multidrug-resistant Shigella sonnei lineage among international Orthodox Jewish communities. Emerg. Infect. Dis. 22, 1545–1553 (2016).
Baker, S. & The, H. C. Recent insights into Shigella: a major contributor to the global diarrhoeal disease burden. Curr. Opin. Infect. Dis. 31, 449–454 (2018).
Bardhan, P., Faruque, A. S. G., Naheed, A. & Sack, D. A. Decreasing shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg. Infect. Dis. 16, 1718–1723 (2010).
Muzembo, B. A. et al. Shigellosis in Southeast Asia: a systematic review and meta-analysis. Travel Med. Infect. Dis. 52, 102554 (2023).
Ibrahim, A. F. et al. The changing epidemiology of shigellosis in Australia, 2001–2019. PLoS Negl. Trop. Dis. 17, e0010450 (2023).
Sabour, S. et al. Molecular detection and characterization of Shigella spp. harboring extended-spectrum β-lactamase genes in children with diarrhea in northwest Iran. Mol. Cell. Pediatr. 9, 19 (2022).
Taneja, N. et al. Antimicrobial resistance in Shigella species: our five years (2015–2019) experience in a tertiary care center in North India. Indian J. Med. Microbiol. 39, 489–494 (2021).
Bardsley, M. et al. Persistent transmission of shigellosis in England is associated with a recently emerged multidrug-resistant strain of Shigella sonnei. J. Clin. Microbiol. https://doi.org/10.1128/jcm.01692-19 (2020).
Mandal, J., Emelda, V. G. J., Mahadevan, S. & Parija, S. C. The recent trends of shigellosis: a JIPMER perspective. J. Clin. Diagn. Res. 6, 1474–1477 (2012).
Taneja, N. et al. Antimicrobial resistant Shigella in North India since the turn of the 21st century. Indian J. Med. Microbiol. 40, 113–118 (2022).
Anandan, S. et al. Molecular characterization of antimicrobial resistance in clinical Shigella isolates during 2014 and 2015: trends South India. Germs 7, 115–122 (2017).
Zhao, L. et al. An 11-year study of shigellosis and Shigella species in Taiyuan, China: active surveillance, epidemic characteristics, and molecular serotyping. J. Infect. Public Health 10, 794–798 (2017).
Livio, S. et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 59, 933–941 (2014).
Kasumba, I. N. et al. Shigella in Africa: new insights from the Vaccine Impact on Diarrhea in Africa (VIDA) study. Clin. Infect. Dis. 76, S66–S76 (2023).
Holt, K. E. et al. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat. Genet. 44, 1056–1059 (2012). One of the first studies that track global spread and evolution of S. sonnei.
Holt, K. E. et al. Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc. Natl Acad. Sci. USA 110, 17522–17527 (2013).
Thompson, C. N., Duy, P. T. & Baker, S. The rising dominance of Shigella sonnei: an intercontinental shift in the etiology of bacillary dysentery. PLoS Negl. Trop. Dis. 9, e0003708 (2015).
Bangtrakulnonth, A. et al. Shigella from humans in Thailand during 1993 to 2006: spatial-time trends in species and serotype distribution. Foodborne Pathog. Dis. 5, 773–784 (2008).
Salah Ud-Din, A. et al. Changing trends in the prevalence of Shigella species: emergence of multi-drug resistant Shigella sonnei biotype g in Bangladesh. PLoS ONE 8, e82601 (2013).
Ram, P. K., Crump, J. A., Gupta, S. K., Miller, M. A. & Mintz, E. D. Part II. Analysis of data gaps pertaining to Shigella infections in low and medium human development index countries, 1984–2005. Epidemiol. Infect. 136, 577–603 (2008).
Vinh, H. et al. A changing picture of shigellosis in Southern Vietnam: shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infect. Dis. 9, 204 (2009).
Baker, K. S. et al. Genomic epidemiology of Shigella in the United Kingdom shows transmission of pathogen sublineages and determinants of antimicrobial resistance. Sci. Rep. 8, 7389 (2018).
The, H. C. & Baker, S. Out of Asia: the independent rise and global spread of fluoroquinolone-resistant Shigella. Microb. Genom. 4, e000171 (2018).
Mason, L. C. E. et al. The evolution and international spread of extensively drug resistant Shigella sonnei. Nat. Commun. 14, 1983 (2023). A recent large-scale global study of the spread of resistant S. sonnei in MSM populations.
Adamker, G. et al. Prediction of shigellosis outcomes in Israel using machine learning classifiers. Epidemiol. Infect. 146, 1445–1451 (2018).
Gupta, A., Polyak, C. S., Bishop, R. D., Sobel, J. & Mintz, E. D. Laboratory-confirmed shigellosis in the United States, 1989–2002: epidemiologic trends and patterns. Clin. Infect. Dis. 38, 1372–1377 (2004).
Ashbaugh, H. R. et al. A multisite network assessment of the epidemiology and etiology of acquired diarrhea among U.S. military and Western travelers (Global Travelers’ Diarrhea Study): a principal role of norovirus among travelers with gastrointestinal illness. Am. J. Trop. Med. Hyg. 103, 1855–1863 (2020).
Shah, N., DuPont, H. L. & Ramsey, D. J. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am. J. Trop. Med. Hyg. 80, 609–614 (2009).
Trépanier, S. et al. Travel-related shigellosis in Quebec, Canada: an analysis of risk factors. J. Travel Med. 21, 304–309 (2014).
Cohen, D. et al. Recent trends in the epidemiology of shigellosis in Israel. Epidemiol. Infect. 142, 2583–2594 (2014).
Cohen, D. et al. Burden and risk factors of Shigella sonnei shigellosis among children aged 0–59 months in hyperendemic communities in Israel. Int. J. Infect. Dis. 82, 117–123 (2019).
Garrett, V. et al. A recurring outbreak of Shigella sonnei among traditionally observant Jewish children in New York City: the risks of daycare and household transmission. Epidemiol. Infect. 134, 1231–1236 (2006).
George, C. M. et al. Shigella infections in household contacts of pediatric shigellosis patients in rural Bangladesh. Emerg. Infect. Dis. 21, 2006–2013 (2015).
Nygren, B. L. et al. Foodborne outbreaks of shigellosis in the USA, 1998–2008. Epidemiol. Infect. 141, 233–241 (2013).
Utsumi, M., Makimoto, K., Quroshi, N. & Ashida, N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing 39, 299–305 (2010).
Simms, I. et al. Intensified shigellosis epidemic associated with sexual transmission in men who have sex with men — Shigella flexneri and S. sonnei in England, 2004 to end of February 2015. Eurosurveillance 20, 21097 (2015).
Tauxe, R. V., McDonald, R. C., Hargrett-Bean, N. & Blake, P. A. The persistence of Shigella flexneri in the United States: increasing role of adult males. Am. J. Public Health 78, 1432–1435 (1988).
Ingle, D. J. et al. Co-circulation of multidrug-resistant Shigella among men who have sex with men in Australia. Clin. Infect. Dis. 69, 1535–1544 (2019).
Charles, H. et al. Outbreak of sexually transmitted, extensively drug-resistant Shigella sonnei in the UK, 2021–22: a descriptive epidemiological study. Lancet Infect. Dis. 22, 1503–1510 (2022).
Lefèvre, S. et al. Rapid emergence of extensively drug-resistant Shigella sonnei in France. Nat. Commun. 14, 462 (2023).
Connor, T. R. et al. Species-wide whole genome sequencing reveals historical global spread and recent local persistence in Shigella flexneri. eLife 4, e07335 (2015).
McVicker, G. & Tang, C. M. Deletion of toxin–antitoxin systems in the evolution of Shigella sonnei as a host-adapted pathogen. Nat. Microbiol. 2, 1–8 (2016).
Cohen, D. et al. Reduction of transmission of shigellosis by control of houseflies (Musca domestica). Lancet 337, 993–997 (1991).
Qiu, S. et al. A Shigella sonnei clone with extensive drug resistance associated with waterborne outbreaks in China. Nat. Commun. 13, 7365 (2022).
Mikhail, A. F. W. et al. Utility of whole-genome sequencing during an investigation of multiple foodborne outbreaks of Shigella sonnei. Epidemiol. Infect. 149, e71 (2021).
Sack, D. A., Hoque, A. T., Huq, A. & Etheridge, M. Is protection against shigellosis induced by natural infection with Plesiomonas shigelloides? Lancet 343, 1413–1415 (1994).
De Silva, P. M. et al. Escherichia coli killing by epidemiologically successful sublineages of Shigella sonnei is mediated by colicins. eBioMedicine 97, 104822 (2023).
Allen, H. et al. Evidence for re-infection and persistent carriage of Shigella species in adult males reporting domestically acquired infection in England. Clin. Microbiol. Infect. 27, 126.e7–126.e13 (2021).
Behar, A. et al. Microevolution and patterns of transmission of Shigella sonnei within cyclic outbreaks shigellosis, Israel. Emerg. Infect. Dis. 24, 1335–1339 (2018).
Pupo, G. M., Lan, R. & Reeves, P. R. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc. Natl Acad. Sci. USA 97, 10567–10572 (2000).
Yang, F. et al. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 33, 6445–6458 (2005).
Lampel, K. A., Formal, S. B. & Maurelli, A. T. A brief history of Shigella. EcoSal Plus https://doi.org/10.1128/ecosalplus.esp-0006-2017 (2018).
Sonne, C. The bacteriology of atoxic dysentery bacilli (paradysentery bacilli). Zent. Bakteriol. Parasitenkd. Infekt. Hyg. 75, 408–456 (1915).
The, H. C. et al. South Asia as a reservoir for the global spread of ciprofloxacin-resistant Shigella sonnei: a cross-sectional study. PLoS Med. 13, e1002055 (2016).
Thanh Duy, P. et al. Commensal Escherichia coli are a reservoir for the transfer of XDR plasmids into epidemic fluoroquinolone-resistant Shigella sonnei. Nat. Microbiol. 5, 256–264 (2020).
Hawkey, J. et al. Global population structure and genotyping framework for genomic surveillance of the major dysentery pathogen, Shigella sonnei. Nat. Commun. 12, 2684 (2021). A global study of the spread of S. sonnei that proposes a new scheme for genotyping and tracking resistance determinants.
The, H. C. et al. Evolutionary histories and antimicrobial resistance in Shigella flexneri and Shigella sonnei in Southeast Asia. Commun. Biol. 4, 1–12 (2021).
Baker, K. S. et al. Whole genome sequencing of Shigella sonnei through PulseNet Latin America and Caribbean: advancing global surveillance of foodborne illnesses. Clin. Microbiol. Infect. 23, 845–853 (2017).
Ekdahl, K. & Andersson, Y. The epidemiology of travel-associated shigellosis — regional risks, seasonality and serogroups. J. Infect. 51, 222–229 (2005).
Gaudreau, C. et al. Clinical and genomic investigation of an International ceftriaxone- and azithromycin-resistant Shigella sonnei cluster among men who have sex with men, Montréal, Canada 2017–2019. Microbiol. Spectr. 10, e02337-21 (2022).
Tansarli, G. S. et al. Genomic reconstruction and directed interventions in a multidrug-resistant shigellosis outbreak in Seattle, WA, USA: a genomic surveillance study. Lancet Infect. Dis. 23, 740–750 (2023).
World Health Organization. WHO bacterial priority pathogens list, 2024 (WHO, 2024).
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019 (US CDC, 2019).
Williams, P. C. M. & Berkley, J. A. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr. Int. Child Health 38, S50–S65 (2018).
World Health Organization. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1 (WHO, 2005).
World Health Organization. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book (WHO, 2022).
Baker, K. S. et al. Horizontal antimicrobial resistance transfer drives epidemics of multiple Shigella species. Nat. Commun. 9, 1462 (2018).
Ranjbar, R. & Farahani, A. Shigella: antibiotic-resistance mechanisms and new horizons for treatment. Infect. Drug Resist. 12, 3137–3167 (2019).
Turner, S. A., Luck, S. N., Sakellaris, H., Rajakumar, K. & Adler, B. Molecular epidemiology of the SRL pathogenicity island. Antimicrob. Agents Chemother. 47, 727–734 (2003).
Njamkepo, E. et al. Global phylogeography and evolutionary history of Shigella dysenteriae type 1. Nat. Microbiol. 1, 1–10 (2016).
Shakya, G., Acharya, J., Adhikari, S. & Rijal, N. Shigellosis in Nepal: 13 years review of nationwide surveillance. J. Health Popul. Nutr. 35, 36 (2016).
Rajpara, N. et al. Molecular analysis of multidrug resistance in clinical isolates of Shigella spp. from 2001–2010 in Kolkata, India: role of integrons, plasmids, and topoisomerase mutations. Infect. Drug Resist. 11, 87–102 (2018).
Bowen, A. et al. Importation and domestic transmission of Shigella sonnei resistant to ciprofloxacin — United States, May 2014–February 2015. Morb. Mortal. Wkly. Rep. 64, 318–320 (2015).
The, H. C. et al. Dissecting the molecular evolution of fluoroquinolone-resistant Shigella sonnei. Nat. Commun. 10, 4828 (2019).
Boumghar-Bourtchai, L. et al. Macrolide-resistant Shigella sonnei. Emerg. Infect. Dis. 14, 1297–1299 (2008).
Darton, T. C. et al. Azithromycin resistance in Shigella spp. in Southeast Asia. Antimicrob. Agents Chemother. https://doi.org/10.1128/aac.01748-17 (2018).
Locke, R. K., Greig, D. R., Jenkins, C., Dallman, T. J. & Cowley, L. A. Acquisition and loss of CTX-M plasmids in Shigella species associated with MSM transmission in the UK. Microb. Genom. 7, 000644 (2021).
Thorley, K. et al. Emergence of extensively drug-resistant and multidrug-resistant Shigella flexneri serotype 2a associated with sexual transmission among gay, bisexual, and other men who have sex with men, in England: a descriptive epidemiological study. Lancet Infect. Dis. 23, 732–739 (2023).
O’Flanagan, H., Siddiq, M., Llewellyn, C. & Richardson, D. Antimicrobial resistance in sexually transmitted Shigella in men who have sex with men: a systematic review. Int. J. STD AIDS https://doi.org/10.1177/09564624231154942 (2023). A study that reviews drug resistance in MSM.
Rowlinson, E., Berzkalns, A., Thibault, C., Golden, M. & Barbee, L. High incidence of antimicrobial use and overuse in cisgender men who have sex with men at risk of bacterial STIs. Sex. Transm. Infect. 97, A48–A49 (2021).
Baker, K. S. et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect. Dis. 15, 913–921 (2015). One of the first studies to track global transmission of resistant Shigella.
Trivett, H. Increase in extensively drug resistant Shigella sonnei in Europe. Lancet Microb. 3, e481 (2022).
Malaka De Silva, P. et al. A tale of two plasmids: contributions of plasmid associated phenotypes to epidemiological success among Shigella. Proc. R. Soc. B Biol. Sci. 289, 20220581 (2022).
Schnupf, P. & Sansonetti, P. J. Shigella pathogenesis: new insights through advanced methodologies. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.bai-0023-2019 (2019). A study that provides a comprehensive review on Shigella pathogenesis.
Sansonetti, P. J., Kopecko, D. J. & Formal, S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect. Immun. 34, 75–83 (1981).
Mahmoud, R. Y. et al. The multivalent adhesion molecule SSO1327 plays a key role in Shigella sonnei pathogenesis. Mol. Microbiol. 99, 658–673 (2016).
Watson, J. L. et al. Shigella sonnei O-antigen inhibits internalization, vacuole escape, and inflammasome activation. mBio 10, e02654-19 (2019). A study that identifies differences in host cell interactions between S. sonnei and S. flexneri.
Caboni, M. et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 11, e1004749 (2015).
Anderson, M. C., Vonaesch, P., Saffarian, A., Marteyn, B. S. & Sansonetti, P. J. Shigella sonnei encodes a functional T6SS used for interbacterial competition and niche occupancy. Cell Host Microbe 21, 769–776.e3 (2017).
Leung, P. B. et al. Shigella sonnei utilises colicins during inter-bacterial competition. Microbiology 170, 001434 (2024).
Henderson, I. R., Czeczulin, J., Eslava, C., Noriega, F. & Nataro, J. P. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67, 5587–5596 (1999).
Navarro-Garcia, F. et al. Pic, an autotransporter protein secreted by different pathogens in the Enterobacteriaceae family, is a potent mucus secretagogue. Infect. Immun. 78, 4101–4109 (2010).
Ruiz-Perez, F. et al. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc. Natl Acad. Sci. USA 108, 12881–12886 (2011).
Al-Hasani, K., Navarro-Garcia, F., Huerta, J., Sakellaris, H. & Adler, B. The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells. PLoS ONE 4, e8223 (2009).
Benjelloun-Touimi, Z., Sansonetti, P. J. & Parsot, C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17, 123–135 (1995).
Al-Hasani, K. et al. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68, 2457–2463 (2000).
Gu, B. et al. Existence of virulence genes in clinical Shigella sonnei isolates from Jiangsu Province of China: a multicenter study. Ann. Transl. Med. 7, 305 (2019).
Moosavian, M., Ghaderiyan, G. H., Shahin, M. & Navidifar, T. First investigation of the presence of SPATE genes in Shigella species isolated from children with diarrhea infection in Ahvaz, Southwest Iran. IDR 12, 795–804 (2019).
Wenzel, H. et al. Improving chances for successful clinical outcomes with better preclinical models. Vaccine 35, 6798–6802 (2017).
Alphonse, N. & Odendall, C. Animal models of shigellosis: a historical overview. Curr. Opin. Immunol. 85, 102399 (2023).
Willis, A. R. et al. Shigella-induced emergency granulopoiesis protects zebrafish larvae from secondary infection. mBio https://doi.org/10.1128/mbio.00933-18 (2018).
Gomes, M. C., Brokatzky, D., Bielecka, M. K., Wardle, F. C. & Mostowy, S. Shigella induces epigenetic reprogramming of zebrafish neutrophils. Sci. Adv. 9, eadf9706 (2023).
Willis, A. R. et al. Injections of predatory bacteria work alongside host immune cells to treat Shigella infection in zebrafish larvae. Curr. Biol. 26, 3343–3351 (2016).
Torraca, V. et al. Shigella sonnei infection of zebrafish reveals that O-antigen mediates neutrophil tolerance and dysentery incidence. PLoS Pathog. 15, e1008006 (2019).
Islam, D. et al. Evaluation of an intragastric challenge model for Shigella dysenteriae 1 in rhesus monkeys (Macaca mulatta) for the pre-clinical assessment of Shigella vaccine formulations. APMIS 122, 463–475 (2014).
Kinsey, M. D., Formal, S. B., Dammin, G. J. & Giannella, R. A. Fluid and electrolyte transport in rhesus monkeys challenged intracecally with Shigella flexneri 2a. Infect. Immun. 14, 368–371 (1976).
Kuehl, C. J., D’Gama, J. D., Warr, A. R. & Waldor, M. K. An oral inoculation infant rabbit model for Shigella infection. mBio https://doi.org/10.1128/mbio.03105-19 (2020).
Mitchell, P. S. et al. NAIP–NLRC4-deficient mice are susceptible to shigellosis. eLife 9, e59022 (2020). A study that focuses on efforts to develop a suitable mouse model of shigellosis.
Rout, W. R., Formal, S. B., Giannella, R. A. & Dammin, G. J. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology 68, 270–278 (1975).
Shim, D.-H. et al. New animal model of shigellosis in the guinea pig: its usefulness for protective efficacy studies. J. Immunol. 178, 2476–2482 (2007).
Skerniskyte, J. et al. Ascorbate deficiency increases progression of shigellosis in guinea pigs and mice infection models. Gut Microbes 15, 2271597 (2023).
Alphonse, N. et al. A family of conserved bacterial virulence factors dampens interferon responses by blocking calcium signaling. Cell 185, 2354–2369.e17 (2022).
Brunner, K., Samassa, F., Sansonetti, P. J. & Phalipon, A. Shigella-mediated immunosuppression in the human gut: subversion extends from innate to adaptive immune responses. Hum. Vaccines Immunother. 15, 1317–1325 (2019).
Shaughnessy, H. J., Olsson, R. C., Bass, K., Friewer, F. & Levinson, S. O. Experimental human bacillary dysentery: polyvalent dysentery vaccine in its prevention. J. Am. Med. Assoc. 132, 362–368 (1946).
Clarkson, K. A. et al. Immune response characterization after controlled infection with lyophilized Shigella sonnei 53G. mSphere 5, e00988-19 (2020).
Clarkson, K. A. et al. Shigella-specific immune profiles induced after parenteral immunization or oral challenge with either Shigella flexneri 2a or Shigella sonnei. mSphere 6, e00122–21 (2021). An analysis of human challenge studies comparing immune responses to S. sonnei and S. flexneri.
Grassart, A. et al. Bioengineered human organ-on-chip reveals intestinal microenvironment and mechanical forces impacting Shigella infection. Cell Host Microbe 26, 435–444.e4 (2019).
Boquet-Pujadas, A. et al. 4D live imaging and computational modeling of a functional gut-on-a-chip evaluate how peristalsis facilitates enteric pathogen invasion. Sci. Adv. 8, eabo5767 (2022).
Ranganathan, S., Smith, E. M., Foulke-Abel, J. D. & Barry, E. M. Research in a time of enteroids and organoids: how the human gut model has transformed the study of enteric bacterial pathogens. Gut Microbes 12, 1795389 (2020).
Perlman, M., Senger, S., Verma, S., Carey, J. & Faherty, C. S. A foundational approach to culture and analyze malnourished organoids. Gut Microbes 15, 2248713 (2023).
Ranganathan, S. et al. Evaluating Shigella flexneri pathogenesis in the human enteroid model. Infect. Immun. https://doi.org/10.1128/iai.00740-18 (2019).
Chanin, R. B. et al. Shigella flexneri adherence factor expression in in vivo-like conditions. mSphere https://doi.org/10.1128/msphere.00751-19 (2019).
Anderson, M., Sansonetti, P. J. & Marteyn, B. S. Shigella diversity and changing landscape: insights for the twenty-first century. Front. Cell. Infect. Microbiol. https://doi.org/10.3389/fcimb.2016.00045 (2016).
Calcuttawala, F., Hariharan, C., Pazhani, G. P., Ghosh, S. & Ramamurthy, T. Activity spectrum of colicins produced by Shigella sonnei and genetic mechanism of colicin resistance in conspecific S. sonnei strains and Escherichia coli. Antimicrob. Agents Chemother. 59, 152–158 (2014).
The, H. C. et al. Assessing gut microbiota perturbations during the early phase of infectious diarrhea in Vietnamese children. Gut Microbes 9, 38–54 (2018).
Ndungo, E. et al. Dynamics of the gut microbiome in Shigella-infected children during the first two years of life. mSystems 7, e00442-22 (2022).
Girardin, S. E. et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003).
Ogawa, M. et al. Escape of intracellular Shigella from autophagy. Science 307, 727–731 (2005).
Mostowy, S. et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 8, 433–444 (2010).
Li, P. et al. Ubiquitination and degradation of GBPs by a Shigella effector to suppress host defence. Nature 551, 378–383 (2017).
Wandel, M. P. et al. GBPs inhibit motility of Shigella flexneri but are targeted for degradation by the bacterial ubiquitin ligase IpaH9.8. Cell Host Microbe 22, 507–518.e5 (2017).
Roncaioli, J. L. et al. A hierarchy of cell death pathways confers layered resistance to shigellosis in mice. eLife 12, e83639 (2023).
Rojas-Lopez, M. et al. NLRP11 is a pattern recognition receptor for bacterial lipopolysaccharide in the cytosol of human macrophages. Sci. Immunol. 8, eabo4767 (2023).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Sansonetti, P. J., Arondel, J., Huerre, M., Harada, A. & Matsushima, K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect. Immun. 67, 1471–1480 (1999).
Weinrauch, Y., Drujan, D., Shapiro, S. D., Weiss, J. & Zychlinsky, A. Neutrophil elastase targets virulence factors of enterobacteria. Nature 417, 91–94 (2002).
Mandic-Mulec, I., Weiss, J. & Zychlinsky, A. Shigella flexneri is trapped in polymorphonuclear leukocyte vacuoles and efficiently killed. Infect. Immun. 65, 110–115 (1997).
François, M., Le Cabec, V., Dupont, M.-A., Sansonetti, P. J. & Maridonneau-Parini, I. Induction of necrosis in human neutrophils by Shigella flexneri requires type III secretion, IpaB and IpaC invasins, and actin polymerization. Infect. Immun. 68, 1289–1296 (2000).
Ciancarella, V. et al. Role of a fluid-phase PRR in fighting an intracellular pathogen: PTX3 in Shigella infection. PLoS Pathog. 14, e1007469 (2018).
Meron-Sudai, S. et al. Pentraxin 3 and Shigella LPS and IpaB antibodies interplay to defeat shigellosis. J. Clin. Med. 11, 4384 (2022).
Nothelfer, K. et al. B lymphocytes undergo TLR2-dependent apoptosis upon Shigella infection. J. Exp. Med. 211, 1215–1229 (2014).
Pinaud, L. et al. Injection of T3SS effectors not resulting in invasion is the main targeting mechanism of Shigella toward human lymphocytes. Proc. Natl Acad. Sci. USA 114, 9954–9959 (2017).
Pinaud, L., Sansonetti, P. J. & Phalipon, A. Host cell targeting by enteropathogenic bacteria T3SS effectors. Trends Microbiol. 26, 266–283 (2018).
Frenck, R. W. et al. Establishment of a controlled human infection model with a lyophilized strain of Shigella sonnei 53G. mSphere 5, e00416–e00420 (2020).
MacLennan, C. A., Grow, S., Ma, L. & Steele, A. D. The Shigella vaccines pipeline. Vaccines 10, 1376 (2022). A paper that reviews the current status of vaccines in development against Shigella.
MacLennan, C. A. & Steele, A. D. Frontiers in Shigella vaccine development. Vaccines 10, 1536 (2022).
DuPont, H. L. et al. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J. Infect. Dis. 125, 12–16 (1972).
Herrington, D. A. et al. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi–Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 8, 353–357 (1990).
Formal, S. B. et al. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 164, 533–537 (1991).
Cohen, D. et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 349, 155–159 (1997). To our knowledge, the first clinical trial of a conjugate Shigella vaccine.
Cohen, D. et al. Serum IgG antibodies to Shigella lipopolysaccharide antigens — a correlate of protection against shigellosis. Hum. Vaccin. Immunother. 15, 1401–1408 (2019).
Cohen, D. et al. Threshold protective levels of serum IgG to Shigella lipopolysaccharide: re-analysis of Shigella vaccine trials data. Clin. Microbiol. Infect. 29, 366–371 (2023).
Plotkin, S. A. & Gilbert, P. B. Nomenclature for immune correlates of protection after vaccination. Clin. Infect. Dis. 54, 1615–1617 (2012).
Robbins, J. B., Chu, C. & Schneerson, R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin. Infect. Dis. 15, 346–361 (1992).
Robbins, J. B., Schneerson, R. & Szu, S. C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 171, 1387–1398 (1995).
Cohen, D. et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: a phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 21, 546–558 (2021).
de Alwis, R. et al. The identification of novel immunogenic antigens as potential Shigella vaccine components. Genome Med. 13, 8 (2021).
McGuire, M. K. et al. Multipathogen analysis of IgA and IgG antigen specificity for selected pathogens in milk produced by women from diverse geographical regions: the INSPIRE study. Front. Immunol. 11, 614372 (2020).
Bernshtein, B. et al. Systems approach to define humoral correlates of immunity to Shigella. Cell Rep. 40, 111216 (2022).
Bernshtein, B. et al. Determinants of immune responses predictive of protection against shigellosis in an endemic zone: a systems analysis of antibody profiles and function. Lancet Microbe 5, 100889 (2024).
Raso, M. M., Arato, V., Gasperini, G. & Micoli, F. Toward a Shigella vaccine: opportunities and challenges to fight an antimicrobial-resistant pathogen. Int. J. Mol. Sci. 24, 4649 (2023).
Wellcome Trust, Boston Consulting Group. Vaccines to Tackle Drug Resistant Infections: An Evaluation of R&D Opportunities (BCG, 2021).
World Health Organization. WHO Preferred Product Characteristics for Vaccines Against Shigella (WHO, 2021).
World Health Organization. Full Value of Vaccine Assessment for Shigella Vaccines (WHO, 2020).
Gavi. Vaccine investment strategy 2024. Gavi, The Vaccine Alliance https://www.gavi.org/our-alliance/strategy/vaccine-investment-strategy-2024 (2023).
Gerke, C. et al. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS ONE 10, e0134478 (2015).
Riddle, M. S., Chen, W. H., Kirkwood, C. D. & MacLennan, C. A. Update on vaccines for enteric pathogens. Clin. Microbiol. Infect. 24, 1039–1045 (2018).
Talaat, K. R. et al. Consensus report on Shigella controlled human infection model: conduct of studies. Clin. Infect. Dis. 69, S580–S590 (2019).
Porter, C. K., Thura, N., Ranallo, R. T. & Riddle, M. S. The Shigella human challenge model. Epidemiol. Infect. 141, 223–232 (2013).
Giersing, B. K. et al. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine 37, 4778–4783 (2019).
Frenck, R. W. et al. A phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine 36, 4880–4889 (2018).
Mo, Y. et al. Safety and immunogenicity of a Shigella bivalent conjugate vaccine (ZF0901) in 3-month- to 5-year-old children in China. Vaccines 10, 33 (2022).
Micoli, F., Nakakana, U. N. & Berlanda Scorza, F. Towards a four-component GMMA-based vaccine against Shigella. Vaccines 10, 328 (2022).
Rossi, O. et al. A next-generation GMMA-based vaccine candidate to fight shigellosis. npj Vaccines 8, 1–10 (2023).
Yang, J. et al. Revisiting the molecular evolutionary history of Shigella spp. J. Mol. Evol. 64, 71–79 (2007).
Sahl, J. W. et al. Defining the phylogenomics of Shigella species: a pathway to diagnostics. J. Clin. Microbiol. 53, 951–960 (2015).
Hawkey, J., Monk, J. M., Billman-Jacobe, H., Palsson, B. & Holt, K. E. Impact of insertion sequences on convergent evolution of Shigella species. PLoS Genet. 16, e1008931 (2020).
Sansonetti, P. J., Arondel, J., Cantey, J. R., Prévost, M. C. & Huerre, M. Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect. Immun. 64, 2752–2764 (1996).
Jensen, V. B., Harty, J. T. & Jones, B. D. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer’s patches. Infect. Immun. 66, 3758–3766 (1998).
Bennett, K. M. et al. Induction of colonic M cells during intestinal inflammation. Am. J. Pathol. 186, 1166–1179 (2016).
Arena, E. T. et al. Bioimage analysis of Shigella infection reveals targeting of colonic crypts. Proc. Natl Acad. Sci. USA 112, E3282–E3290 (2015).
Nigro, G. et al. Mapping of Shigella flexneri’s tissue distribution and type III secretion apparatus activity during infection of the large intestine of guinea pigs. Pathog. Dis. 77, ftz054 (2019).
Ashida, H., Suzuki, T. & Sasakawa, C. Shigella infection and host cell death: a double-edged sword for the host and pathogen survival. Curr. Opin. Microbiol. 59, 1–7 (2021).
Lowell, G. H. et al. Antibody-dependent cell-mediated antibacterial activity: K lymphocytes, monocytes, and granulocytes are effective against Shigella. J. Immunol. 125, 2778–2784 (1980).
Hosangadi, D., Smith, P. G. & Giersing, B. K. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine 37, 7372–7380 (2019).
Murray, C. J. L. et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223 (2012).
Liu, J. et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388, 1291–1301 (2016).
Author information
Authors and Affiliations
Contributions
T.A.S. and K.S.B. researched data for article. T.A.S., K.S.B., C.T., S.M. and J.H. substantially contributed to the discussion of content. T.A.S., K.S.B., C.T., S.M. and H.S. wrote the article. T.A.S., K.S.B., C.T., C.J., S.M., J.H., H.S., K.E.H., N.R.T. and S.B. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Benoit Marteyn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scott, T.A., Baker, K.S., Trotter, C. et al. Shigella sonnei: epidemiology, evolution, pathogenesis, resistance and host interactions. Nat Rev Microbiol 23, 303–317 (2025). https://doi.org/10.1038/s41579-024-01126-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-024-01126-x
This article is cited by
-
Organoid models advance dysentery control
Nature Genetics (2025)