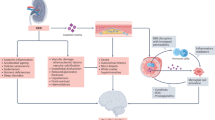
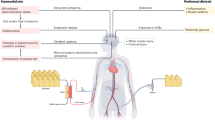
Abstract
Ageing populations worldwide face increasing challenges of multimorbidity (that is, the co-occurrence of two or more chronic conditions). The combination of chronic kidney disease (CKD) and dementia occurs more frequently than it would by simple coincidence, owing to several underlying biological and clinical mechanisms. Population-based cohort studies are an important epidemiological tool and have contributed to improved understanding of these mechanisms. These mechanisms include uniquely shared haemodynamic features of vasculature, overlapping risk factor profiles, and direct neurotoxic effects of accumulating waste products due to poor kidney function. The effect of these pathways is suggested to differ across gender, relevant demographic subgroups, and populations from low- to middle-income countries. Yet, given their study design, population-based cohort studies also inherently face several methodological challenges. These challenges pertain to the use of biomarkers that do not always fully capture the structure and function of the kidney or the brain; bidirectionality across the pathways under study; and practical issues of proper causal inference in light of incomplete distinction between confounders, mediators and effect modifiers. This Review describes our current understanding of the link between CKD and dementia, with a focus on knowledge synthesized from population-based cohort studies. Methodological challenges and possible solutions will be described and directions for future research areas will be outlined.
Key points
-
Chronic kidney disease (CKD) and dementia are frequently co-occurring conditions that pose a large burden on societies worldwide; both conditions have a long subclinical phase that is captured through various biomarkers.
-
Various mechanisms link CKD with dementia, pointing towards possible targets for prevention, but methodological challenges exist.
-
A concerted effort involving caregivers, methodologists, patients and policymakers is required to tackle the clinical and methodological challenges posed by CKD and dementia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Diseases, G. B. D. & Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1204–1222 (2020).
Chi, H. C. et al. Adult renal dysfunction and risk of dementia or cognitive decline: brain-kidney axis hypothesis based on a systematic review and meta-analysis. J. Prev. Alzheimers Dis. 10, 443–452 (2023).
Licher, S. et al. Lifetime risk and multimorbidity of non-communicable diseases and disease-free life expectancy in the general population: a population-based cohort study. PLoS Med. 16, e1002741 (2019).
Jadoul, M., Aoun, M. & Masimango Imani, M. The major global burden of chronic kidney disease. Lancet Glob. Health 12, e342–e343 (2024).
Kidney Disease: Improving Global Outcomes, CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105, S117–S314 (2024).
GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. 23, 344–381 (2024).
Jack, C. R. Jr. et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s association workgroup. Alzheimers Dement. 20, 5143–5169 (2024).
Petersen, R. C. et al. A new framework for dementia nomenclature. JAMA Neurol. 80, 1364–1370 (2023).
Dubois, B. et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the international working group. Lancet Neurol. 20, 484–496 (2021).
Farrell, D. R. & Vassalotti, J. A. Screening, identifying, and treating chronic kidney disease: why, who, when, how, and what? BMC Nephrol. 25, 34 (2024).
Andrassy, K. M. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 84, 622–623 (2013).
Vaidya, S. R., Aeddula, N. R. & Doerr, C. Chronic kidney disease (nursing). In StatPearls https://www.ncbi.nlm.nih.gov/books/NBK568778/ (2024).
Bello, A. K. et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob. Health 12, e382–e395 (2024).
GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public. Health 7, e105–e125 (2022).
Livingston, G. et al. Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet 404, 572–628 (2024).
Wolters, F. J. et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer cohorts consortium. Neurology 95, e519–e531 (2020).
Andersson, C., Johnson, A. D., Benjamin, E. J., Levy, D. & Vasan, R. S. 70-year legacy of the Framingham Heart Study. Nat. Rev. Cardiol. 16, 687–698 (2019).
Mittelmark, M. B. et al. Prevalence of cardiovascular diseases among older adults. Cardiovascular health study. Am. J. Epidemiol. 137, 311–317 (1993).
Ikram, M. A. et al. The Rotterdam study. Design update and major findings between 2020 and 2024. Eur. J. Epidemiol. 39, 183–206 (2024).
The Atherosclerosis Risk In Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol 129, 687–702 (1989).
Bild, D. E. et al. Multi-ethnic study of atherosclerosis: objectives and design. Am. J. Epidemiol. 156, 871–881 (2002).
Batty, G. D., Gale, C. R., Kivimäki, M., Deary, I. J. & Bell, S. Comparison of risk factor associations in UK biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ 368, m131 (2020).
Gaziano, J. M. et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 70, 214–223 (2016).
Peters, A. et al. Framework and baseline examination of the German national cohort (NAKO). Eur. J. Epidemiol. 37, 1107–1124 (2022).
Stefanou, E., Tountas, C., Ioannidis, E. & Kole, C. Biomarkers in cardiorenal syndrome, a potential use in precision medicine. J. Nephrol. 37, 2127–2138 (2024).
Provenzano, M. et al. Estimated glomerular filtration rate in observational and interventional studies in chronic kidney disease. J. Nephrol. 37, 573–586 (2024).
Inker, L. A. et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 385, 1737–1749 (2021).
Watanabe, K., Watanabe, T. & Nakayama, M. Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology 44, 184–193 (2014).
Zheng, Y., Ji, B., Chen, S., Zhou, R. & Ni, R. The impact of uremic toxins on Alzheimer’s disease. Curr. Alzheimer Res. 19, 104–118 (2022).
Greenberg, J. H. et al. Urine biomarkers of kidney tubule health, injury, and inflammation are associated with progression of CKD in children. J. Am. Soc. Nephrol. 32, 2664–2677 (2021).
Zhao, K., Seeliger, E., Niendorf, T. & Liu, Z. Noninvasive assessment of diabetic kidney disease with MRI: hype or hope? J. Magn. Reson. Imaging 59, 1494–1513 (2024).
Singla, R. K., Kadatz, M., Rohling, R. & Nguan, C. Kidney ultrasound for nephrologists: a review. Kidney Med. 4, 100464 (2022).
Scheltens, P. et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021).
Darweesh, S. K. L. et al. Quantitative gait, cognitive decline, and incident dementia: the Rotterdam study. Alzheimers Dement. 15, 1264–1273 (2019).
Wolters, F. J. et al. Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med. 13, e1002143 (2016).
Mooldijk, S. S. & Ikram, M. A. Cerebral small vessel disease in population-based research: what are we looking at — and what not? Aging Dis. 15, 1438–1446 (2024).
Jack, C. R. Jr Advances in Alzheimer’s disease research over the past two decades. Lancet Neurol. 21, 866–869 (2022).
Hainsworth, A. H., Markus, H. S. & Schneider, J. A. Cerebral small vessel disease, hypertension, and vascular contributions to cognitive impairment and dementia. Hypertension 81, 75–86 (2024).
Tang, X. et al. Association of kidney function and brain health: a systematic review and meta-analysis of cohort studies. Ageing Res. Rev. 82, 101762 (2022).
Mund, M. & Nestler, S. Beyond the cross-lagged panel model: next-generation statistical tools for analyzing interdependencies across the life course. Adv. Life Course Res. 41, 100249 (2019).
Xie, Z., Tong, S., Chu, X., Feng, T. & Geng, M. Chronic kidney disease and cognitive impairment: the kidney-brain axis. Kidney Dis. 8, 275–285 (2022).
Kelly, D. M. & Rothwell, P. M. Disentangling the relationship between chronic kidney disease and cognitive disorders. Front. Neurol. 13, 830064 (2022).
Bossola, M. & Picconi, B. Uremic toxins and the brain in chronic kidney disease. J. Nephrol. 37, 1391–1395 (2024).
Lu, R., Kiernan, M. C., Murray, A., Rosner, M. H. & Ronco, C. Kidney-brain crosstalk in the acute and chronic setting. Nat. Rev. Nephrol. 11, 707–719 (2015).
Seliger, S. L., Wendell, C. R., Waldstein, S. R., Ferrucci, L. & Zonderman, A. B. Renal function and long-term decline in cognitive function: the Baltimore Longitudinal Study of Aging. Am. J. Nephrol. 41, 305–312 (2015).
Lee, S. et al. The association between kidney function and cognitive decline in community-dwelling, elderly Japanese people. J. Am. Med. Dir. Assoc. 16, 349.e1–5 (2015).
Berger, I. et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med. 14, 206 (2016).
Bugnicourt, J. M., Godefroy, O., Chillon, J. M., Choukroun, G. & Massy, Z. A. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J. Am. Soc. Nephrol. 24, 353–363 (2013).
Kelly, D. M., et al. Impaired kidney function, cerebral small vessel disease and cognitive disorders: the Framingham Heart Study. Nephrol. Dial. Transplant. 39, 1911–1922 (2024).
Sedaghat, S. et al. The AGES-Reykjavik study suggests that change in kidney measures is associated with subclinical brain pathology in older community-dwelling persons. Kidney Int. 94, 608–615 (2018).
Sedaghat, S. et al. Kidney function and microstructural integrity of brain white matter. Neurology 85, 154–161 (2015).
Scheppach, J. B. et al. Association of kidney function measures with signs of neurodegeneration and small vessel disease on brain magnetic resonance imaging: the Atherosclerosis Risk In Communities (ARIC) study. Am. J. Kidney Dis. 81, 261–269.e1 (2023).
Akoudad, S. et al. Kidney function and cerebral small vessel disease in the general population. Int. J. Stroke 10, 603–608 (2015).
Sedaghat, S. et al. Kidney function and cerebral blood flow: the Rotterdam study. J. Am. Soc. Nephrol. 27, 715–721 (2016).
Seliger, S. L. et al. Cystatin C and subclinical brain infarction. J. Am. Soc. Nephrol. 16, 3721–3727 (2005).
Farrah, T. E., Dhillon, B., Keane, P. A., Webb, D. J. & Dhaun, N. The eye, the kidney, and cardiovascular disease: old concepts, better tools, and new horizons. Kidney Int. 98, 323–342 (2020).
Toyoda, K. Cerebral small vessel disease and chronic kidney disease. J. Stroke 17, 31–37 (2015).
Yan, Q. et al. Kidney–brain axis in the pathogenesis of cognitive impairment. Neurobiol. Dis. 200, 106626 (2024).
Viggiano, D. et al. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 16, 452–469 (2020).
Viggiano, D. et al. Mild cognitive impairment and kidney disease: clinical aspects. Nephrol. Dial. Transpl. 35, 10–17 (2020).
Kang, D. H. et al. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 13, 806–816 (2002).
O’Rourke, M. F. & Safar, M. E. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46, 200–204 (2005).
Kurella Tamura, M. et al. Kidney disease, intensive hypertension treatment, and risk for dementia and mild cognitive impairment: the systolic blood pressure intervention trial. J. Am. Soc. Nephrol. 31, 2122–2132 (2020).
Babroudi, S. et al. Blood pressure, incident cognitive impairment, and severity of CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am. J. Kidney Dis. 82, 443–453.e1 (2023).
Kim, E. D. et al. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: the Atherosclerosis Risk In Communities (ARIC) study. Am. J. Kidney Dis. 72, 682–690 (2018).
Zierler, R. E. et al. Carotid and lower extremity arterial disease in patients with renal artery atherosclerosis. Arch. Intern. Med. 158, 761–767 (1998).
Miralles, M. et al. Screening for carotid and renal artery stenoses in patients with aortoiliac disease. Ann. Vasc. Surg. 12, 17–22 (1998).
Wong, T. Y. et al. Retinal microvascular abnormalities and renal dysfunction: the Atherosclerosis Risk In Communities study. J. Am. Soc. Nephrol. 15, 2469–2476 (2004).
Fernando, M. S. et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 37, 1391–1398 (2006).
Theuerle, J. D. et al. Retinal microvascular function predicts chronic kidney disease in patients with cardiovascular risk factors. Atherosclerosis 341, 63–70 (2022).
Qureshi, A. I. & Caplan, L. R. Intracranial atherosclerosis. Lancet 383, 984–998 (2014).
Sacco, R. L., Kargman, D. E., Gu, Q. & Zamanillo, M. C. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke 26, 14–20 (1995).
Wong, L. K. Global burden of intracranial atherosclerosis. Int. J. Stroke 1, 158–159 (2006).
Gorelick, P. B., Wong, K. S., Bae, H. J. & Pandey, D. K. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 39, 2396–2399 (2008).
Gorelick, P. B. et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713 (2011).
Pajewski, N. M. et al. The legacy effect of intensive versus standard BP control on the incidence of needing dialysis or kidney transplantation. J. Am. Soc. Nephrol. 35, 1737–1745 (2024).
Syrjanen, J. A. et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 18, 1128–1140 (2022).
Sedaghat, S. et al. The association of kidney function with plasma amyloid-β levels and brain amyloid deposition. J. Alzheimers Dis. 92, 229–239 (2023).
Sarto, J. et al. Impact of demographics and comorbid conditions on plasma biomarkers concentrations and their diagnostic accuracy in a memory clinic cohort. J. Neurol. 271, 1973–1984 (2024).
Dittrich, A. et al. Association of chronic kidney disease with plasma NfL and other biomarkers of neurodegeneration: the H70 Birth Cohort Study in Gothenburg. Neurology 101, e277–e288 (2023).
Wu, J. et al. The impact of kidney function on plasma neurofilament light and phospho-tau 181 in a community-based cohort: the Shanghai Aging Study. Alzheimers Res. Ther. 16, 32 (2024).
Adesso, S. et al. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front. Pharmacol. 8, 370 (2017).
Lipton, S. A. et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl Acad. Sci. USA 94, 5923–5928 (1997).
GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249 (2020).
Stefan, N. & Schulze, M. B. Metabolic health and cardiometabolic risk clusters: implications for prediction, prevention, and treatment. Lancet Diabetes Endocrinol. 11, 426–440 (2023).
Nilsson, P. M., Tuomilehto, J. & Rydén, L. The metabolic syndrome — what is it and how should it be managed? Eur. J. Prev. Cardiol. 26, 33–46 (2019).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
Kjaergaard, A. D. et al. Kidney function and risk of dementia: observational study, meta-analysis, and two-sample mendelian randomization study. Eur. J. Epidemiol. 37, 1273–1284 (2022).
Zhao, H., Yuan, H. & Wang, E. Causal effects of kidney function and chronic kidney disease on Alzheimer’s disease by analyzing large-scale genome-wide association study datasets. J. Alzheimers Dis. 102, 321–328 (2024).
Marini, S. et al. Genetic overlap and causal inferences between kidney function and cerebrovascular disease. Neurology 94, e2581–e2591 (2020).
Pohl, D. J. et al. Relationship between residential segregation, later-life cognition, and incident dementia across race/ethnicity. Int. J. Env. Res. Public. Health 18, 11233 (2021).
Lee, H., Caldwell, J. T., Maene, C., Cagney, K. A. & Saunders, M. R. Racial/ethnic inequities in access to high-quality dialysis treatment in Chicago: does neighborhood racial/ethnic composition matter? J. Racial Ethn. Health Disparities 7, 854–864 (2020).
Park, S. et al. Association between visit-to-visit blood pressure variability and risks of dementia in CKD patients: a nationwide observational cohort study. Clin. Kidney J. 15, 1506–1513 (2022).
Drew, D. A. et al. Blood pressure and cognitive decline in prevalent hemodialysis patients. Am. J. Nephrol. 49, 460–469 (2019).
Olczyk, P., Kusztal, M., Gołębiowski, T., Letachowicz, K. & Krajewska, M. Cognitive impairment in end stage renal disease patients undergoing hemodialysis: markers and risk factors. Int. J. Env. Res. Public. Health 19, 2389 (2022).
Yang, S. et al. Association between cognitive function and risk of chronic kidney disease: a longitudinal cohort and mendelian randomization study. Mayo Clin. Proc. 99, 1399–1410 (2024).
Hayat, S. A. et al. Understanding the relationship between cognition and death: a within cohort examination of cognitive measures and mortality. Eur. J. Epidemiol. 33, 1049–1062 (2018).
Tanaka, S. & Okusa, M. D. Crosstalk between the nervous system and the kidney. Kidney Int. 97, 466–476 (2020).
Tanaka, S. et al. Vagus nerve stimulation activates two distinct neuroimmune circuits converging in the spleen to protect mice from kidney injury. Proc. Natl Acad. Sci. USA 118, e2021758118 (2021).
Mooldijk, S. S., Labrecque, J. A., Ikram, M. A. & Ikram, M. K. Ratios in regression analyses with causal questions. Am J Epidemiol 194, 311–313 (2025).
Brookhart, M. A. et al. Variable selection for propensity score models. Am. J. Epidemiol. 163, 1149–1156 (2006).
Knottnerus, J. A. & Tugwell, P. Confounding obscures our view, effect modification is part of reality. J. Clin. Epidemiol. 114, v–vi (2019).
Knol, M. J. & VanderWeele, T. J. Recommendations for presenting analyses of effect modification and interaction. Int. J. Epidemiol. 41, 514–520 (2012).
VanderWeele, T. J. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 25, 749–761 (2014).
Ikram, M. A. & VanderWeele, T. J. A proposed clinical and biological interpretation of mediated interaction. Eur. J. Epidemiol. 30, 1115–1118 (2015).
Bos, D. et al. Thyroid function and atrial fibrillation: is there a mediating role for epicardial adipose tissue? Clin. Epidemiol. 10, 225–234 (2018).
Ma, Y. et al. APOE ε4 and late-life cognition: mediation by structural brain imaging markers. Eur. J. Epidemiol. 37, 591–601 (2022).
Berry, D. & Willoughby, M. T. On the practical interpretability of cross-lagged panel models: rethinking a developmental workhorse. Child. Dev. 88, 1186–1206 (2017).
Hamaker, E. L., Kuiper, R. M. & Grasman, R. P. A critique of the cross-lagged panel model. Psychol. Methods 20, 102–116 (2015).
Murray, A. M. & Vemuri, P. Kidney disease and brain health: current knowledge and next steps. Am. J. Kidney Dis. 81, 253–255 (2023).
Mc Causland, F. R. et al. Finerenone and kidney outcomes in patients with heart failure: the FINEARTS-HF trial. J. Am. Coll. Cardiol. 85, 159–168 (2025).
Zheng, S. L. et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA 319, 1580–1591 (2018).
Sohn, M., Dietrich, J. W., Nauck, M. A. & Lim, S. Characteristics predicting the efficacy of SGLT-2 inhibitors versus GLP-1 receptor agonists on major adverse cardiovascular events in type 2 diabetes mellitus: a meta-analysis study. Cardiovasc. Diabetol. 22, 153 (2023).
Sheets, K. M., Davey, C. S., St Peter, W. L., Reule, S. A. & Murray, A. M. Cognitive impairment, perceived medication adherence, and high-risk medication use in patients with reduced kidney function: a cross-sectional analysis. Health Sci. Rep. 5, e697 (2022).
Copur, S., Berkkan, M., Sarafidis, P. & Kanbay, M. Intensive blood pressure control on dementia in patients with chronic kidney disease: potential reduction in disease burden. Eur. J. Intern. Med. 101, 8–13 (2022).
van Buchem, M. A. et al. The heart–brain connection: a multidisciplinary approach targeting a missing link in the pathophysiology of vascular cognitive impairment. J. Alzheimers Dis. 42, S443–S451 (2014).
Nijskens, C. M. et al. Is it time for Heart–Brain clinics? A clinical survey and proposition to improve current care for cognitive problems in heart failure. Clin. Cardiol. 47, e24200 (2024).
Li, Y. et al. Neighborhood racial and ethnic segregation and the risk of dementia in older adults living with kidney failure. J. Am. Soc. Nephrol. 35, 936–948 (2024).
Taubman, S. L., Robins, J. M., Mittleman, M. A. & Hernán, M. A. Intervening on risk factors for coronary heart disease: an application of the parametric g-formula. Int. J. Epidemiol. 38, 1599–1611 (2009).
Young, J. G., Cain, L. E., Robins, J. M., O’Reilly, E. J. & Hernán, M. A. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat. Biosci. 3, 119–143 (2011).
Naimi, A. I., Cole, S. R. & Kennedy, E. H. An introduction to g methods. Int. J. Epidemiol. 46, 756–762 (2017).
VanderWeele, T. J. & Tchetgen Tchetgen, E. J. Mediation analysis with time varying exposures and mediators. J. R. Stat. Soc. Ser. B Stat. Methodol. 79, 917–938 (2017).
Robins, J. M., Hernán, M. A. & Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 11, 550–560 (2000).
Wehbe, R. M. et al. Deep learning for cardiovascular imaging: a review. JAMA Cardiol. 8, 1089–1098 (2023).
Oikonomou, E. K. & Khera, R. Machine learning in precision diabetes care and cardiovascular risk prediction. Cardiovasc. Diabetol. 22, 259 (2023).
Haug, C. J. & Drazen, J. M. Artificial intelligence and machine learning in clinical medicine, 2023. N. Engl. J. Med. 388, 1201–1208 (2023).
Giddings, R. et al. Factors influencing clinician and patient interaction with machine learning-based risk prediction models: a systematic review. Lancet Digit. Health 6, e131–e144 (2024).
VanderWeele, T. J. Principles of confounder selection. Eur. J. Epidemiol. 34, 211–219 (2019).
Lin, S. H. & Ikram, M. A. On the relationship of machine learning with causal inference. Eur. J. Epidemiol. 35, 183–185 (2020).
Feuerriegel, S. et al. Causal machine learning for predicting treatment outcomes. Nat. Med. 30, 958–968 (2024).
Mitra, N., Roy, J. & Small, D. The future of causal inference. Am. J. Epidemiol. 191, 1671–1676 (2022).
Acknowledgements
In the past 5 years, Dr Ikram’s work has been supported by various institutions and funding agencies, including Erasmus University Medical Center, Erasmus Trustfonds, Netherlands Research Council (NWO), Netherlands Organisation for Health Research and Development (ZonMw), Health-Holland, EU Horizon 2020 Programme, European Research Council, EIT Health, Nederlandse Hartstichting, Alzheimer Association, Alzheimer Nederland, Stichting Parkinson Fonds, and Janssen Pharmaceutical Companies of Johnson & Johnson.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Causal inference
-
A framework of conceptual reasoning and analytical approach to strengthen causal conclusions in empirical research.
- Confounder
-
A shared risk factor between the exposure and the outcome. The presence of a confounder can lead to a spurious association between exposure and outcome. (Note that a single variable in a dataset may be a source of either confounding or interaction, or both mediation and interaction at the same time).
- Cross-lagged analyses
-
An analytical approach using repeated measurements, in which several variables are analysed concomitantly, enabling the study of multiple causal directions. For instance, such analyses would not only model variable A causing B, but also model B causing A.
- Effect modification
-
A phenomenon where the effect of an exposure on an outcome varies depending on the level of another variable, called the effect modifier. Effect modifiers can point towards biological interaction and are also referred to as moderators.
- Exposure
-
A factor that is of primary interest in a study as a risk factor or marker. In statistical models, the exposure is often referred to as the independent variable.
- G-methods
-
A set of advanced statistical approaches that enables the analysis of complex longitudinal data, for instance, in the presence of time-varying confounding.
- Interaction
-
A phenomenon where the effect of two exposures together on an outcome is different to the sum of the effects of either exposure separately. This concept is closely linked to effect modification.
- Inverse probability weighting
-
A statistical approach that enables estimation (extrapolation) of variables and results to populations other than the population from which the data were generated.
- Joint longitudinal-survival model
-
A statistical approach that allows jointly modelling longitudinal data (i.e. repeated measurements) with survival data (i.e. time to event). Such an approach would be ideal to model the association of kidney function over time (using repeated measurements) with the risk of dementia.
- Mediation analysis
-
An analytical approach to disentangle (mechanistic) pathways from exposures to outcomes. Typically, an overall effect of exposure on outcome is separated into a direct effect and an indirect effect, the latter pointing towards the effect that goes through a mediator of interest.
- Mediator
-
An intermediate factor that (partly) relays the effect of the exposure on the outcome. Mediators may provide mechanistic insights and identify biological pathways linking exposure to outcome. (Note that a single variable in a dataset may be a source of both confounding and interaction or both mediation and interaction at the same time).
- Reverse causality
-
A phenomenon where the association between two variables is in the opposite causal direction from the presumed direction. For instance, in the association of CKD causing dementia the possibility that dementia may in turn cause CKD is indicative of reverse causality.
- Selective inclusion
-
The situation when the inclusion criteria for a study lead to a discrepancy between the population for which the findings of the study are intended (i.e. target population) and the population that is eligible for the study.
- Selective participation
-
The situation when participation in the study leads to a discrepancy between people who were eligible to participate and those who actually participated.
- Stratified analyses
-
An approach in which a total sample size is separated into subgroups (e.g. men versus women; people with diabetes versus people without diabetes). Statistical analyses are then carried out within these subgroups. These subgroups are often termed strata.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikram, M.A. Chronic kidney disease and dementia: an epidemiological perspective. Nat Rev Nephrol (2025). https://doi.org/10.1038/s41581-025-00967-w
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-025-00967-w