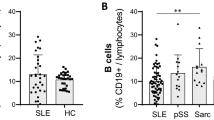
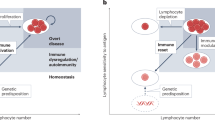
Abstract
B cell depletion with rituximab, a chimeric monoclonal antibody that selectively targets B cells by binding CD20, has been used off label in severe and resistant systemic lupus erythematosus (SLE) for over two decades. Several biological mechanisms limit the efficacy of rituximab, including immunological reactions towards the chimeric molecule, increased numbers of residual B cells, including plasmablasts and plasma cells, and a post-treatment surge in B cell-activating factor (BAFF) levels. Consequently, rituximab induces remission in only a proportion of patients, and safety issues limit its use. However, the use of rituximab has established the value of B cell depletion strategies in SLE and has guided the development of several improved B cell depletion therapies for SLE. These include enhanced monoclonal antibodies, modalities that redirect the specificity of patient T cells using chimeric antigen receptor T cells or bispecific T cell engagers, and combination treatment that simultaneously inhibits the BAFF pathway. In this Review, we consider evidence gathered from over two decades of using rituximab in SLE and examine how B cell depletion therapies could be further optimized to achieve immunological and clinical efficacy. In addition, we discuss the prospects of B cell depletion strategies for personalized treatment in SLE based on genetic research and studies in pre-symptomatic individuals.
Key points
-
Although the B cell depletion agent rituximab failed to reach its primary end points in randomized controlled trials in systemic lupus erythematosus (SLE), favourable clinical experience has led to its frequent off-label use in patients with SLE.
-
Deep B cell depletion of prolonged duration has been associated with improved clinical response to rituximab.
-
Additional B cell depletion therapies that enhance B cell depletion, reduce immunogenicity, delay relapse of B cell numbers or target memory B cells and plasma cells are under development, although trials comparing these therapies head to head are lacking.
-
Innate and non-immune mechanisms that lead to B cell activation, as well as B cell-independent inflammation, might underlie resistance to B cell depletion therapy.
-
Although enhanced B cell depletion improves clinical responses in patients with SLE, both B cell-driven mechanisms and innate or non-immune mechanisms might need to be targeted to achieve cure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 71, 1400–1412 (2019).
Looney, R. J. et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 50, 2580–2589 (2004).
Leandro, M. J., Edwards, J. C., Cambridge, G., Ehrenstein, M. R. & Isenberg, D. A. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 46, 2673–2677 (2002).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Jones, R. B. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 363, 211–220 (2010).
Smith, R. M. et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann. Rheum. Dis. 79, 1243–1249 (2020).
Guillevin, L. et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N. Engl. J. Med. 371, 1771–1780 (2014).
Specks, U. et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N. Engl. J. Med. 369, 417–427 (2013).
Reddy, V. et al. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol. 67, 2046–2055 (2015).
Lim, S. H. et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 118, 2530–2540 (2011).
Cole, S. et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther. 20, 85 (2018).
Clavarino, G. et al. Novel strategy for phenotypic characterization of human B lymphocytes from precursors to effector cells by flow cytometry. PLoS ONE 11, e0162209 (2016).
Álvarez Gómez, J. A. et al. BAFF system expression in double negative 2, activated naïve and activated memory B cells in systemic lupus erythematosus. Front. Immunol. 14, 1235937 (2023).
Rodig, S. J., Shahsafaei, A., Li, B., Mackay, C. R. & Dorfman, D. M. BAFF-R, the major B cell-activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum. Pathol. 36, 1113–1119 (2005).
Tipton, C. M. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16, 755–765 (2015).
Rivero, S. J., Díaz-Jouanen, E. & Alarcón-Segovia, D. Lymphopenia in systemic lupus erythematosus. Clinical, diagnostic, and prognostic significance. Arthritis Rheum. 21, 295–305 (1978).
Dörner, T. & Lipsky, P. E. The essential roles of memory B cells in the pathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 20, 770–782 (2024).
Odendahl, M. et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 165, 5970–5979 (2000).
Liu, M. et al. Type I interferons promote the survival and proinflammatory properties of transitional B cells in systemic lupus erythematosus patients. Cell. Mol. Immunol. 16, 367–379 (2019).
Suurmond, J. et al. Patterns of ANA+ B cells for SLE patient stratification. JCI Insight 4, e127885 (2019).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022).
Suurmond, J. et al. Loss of an IgG plasma cell checkpoint in patients with lupus. J. Allergy Clin. Immunol. 143, 1586–1597 (2019).
Eckl-Dorna, J. & Batista, F. D. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood 113, 3969–3977 (2009).
Lau, C. M. et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 (2005).
Leadbetter, E. A. et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Jenks, S. A. et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49, 725–739.e6 (2018).
Walsh, E. R. et al. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc. Natl Acad. Sci. USA 109, 16276–16281 (2012).
Wei, C. et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 178, 6624–6633 (2007).
Baxter, R. M. et al. Expansion of extrafollicular B and T cell subsets in childhood-onset systemic lupus erythematosus. Front. Immunol. 14, 1208282 (2023).
Sasaki, T. et al. Longitudinal immune cell profiling in patients with early systemic lupus erythematosus. Arthritis Rheumatol. 74, 1808–1821 (2022).
Lam, J. H. & Baumgarth, N. Toll-like receptor mediated inflammation directs B cells towards protective antiviral extrafollicular responses. Nat. Commun. 14, 3979 (2023).
Jacobi, A. M. et al. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 69, 305–308 (2010).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 64, 1215–1226 (2012).
Merrill, J. T. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62, 222–233 (2010).
Reddy, V., Jayne, D., Close, D. & Isenberg, D. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design. Arthritis Res. Ther. 15, S2 (2013).
McCarthy, E. M. et al. Short-term efficacy and safety of rituximab therapy in refractory systemic lupus erythematosus: results from the British Isles Lupus Assessment Group Biologics Register. Rheumatology 57, 470–479 (2018).
Aguiar, R., Araújo, C., Martins-Coelho, G. & Isenberg, D. Use of rituximab in systemic lupus erythematosus: a single center experience over 14 years. Arthritis Care Res. 69, 257–262 (2017).
Díaz-Lagares, C. et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis: pooled data from European cohorts. Autoimmun. Rev. 11, 357–364 (2012).
Lan, L., Han, F. & Chen, J. H. Efficacy and safety of rituximab therapy for systemic lupus erythematosus: a systematic review and meta-analysis. J. Zhejiang Univ. Sci. B 13, 731–744 (2012).
Ramos-Casals, M., Soto, M. J., Cuadrado, M. J. & Khamashta, M. A. Rituximab in systemic lupus erythematosus: a systematic review of off-label use in 188 cases. Lupus 18, 767–776 (2009).
Galarza-Maldonado, C. et al. The administration of low doses of rituximab followed by hydroxychloroquine, prednisone and low doses of mycophenolate mofetil is an effective therapy in Latin American patients with active systemic lupus erythematosus. Autoimmun. Rev. 10, 108–111 (2010).
Vital, E. M. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 63, 3038–3047 (2011).
Turner-Stokes, T. et al. The efficacy of repeated treatment with B-cell depletion therapy in systemic lupus erythematosus: an evaluation. Rheumatology 50, 1401–1408 (2011).
Anolik, J. H. et al. The relationship of FcγRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 48, 455–459 (2003).
Robinson, J. I. et al. Comprehensive genetic and functional analyses of Fc gamma receptors influence on response to rituximab therapy for autoimmunity. EBioMedicine 86, 104343 (2022).
Vital, E. M., Dass, S., Buch, M. H., Rawstron, A. C. & Emery, P. An extra dose of rituximab improves clinical response in rheumatoid arthritis patients with initial incomplete B cell depletion: a randomised controlled trial. Ann. Rheum. Dis. 74, 1195–1201 (2015).
Albert, D. et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann. Rheum. Dis. 67, 1724–1731 (2008).
Gomez Mendez, L. M. et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin. J. Am. Soc. Nephrol. 13, 1502–1509 (2018).
Anolik, J. H. et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 50, 3580–3590 (2004).
Anolik, J. H. et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 56, 3044–3056 (2007).
Md Yusof, M. Y. et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann. Rheum. Dis. 76, 1829–1836 (2017).
Weisel, N. M. et al. Comprehensive analyses of B-cell compartments across the human body reveal novel subsets and a gut-resident memory phenotype. Blood 136, 2774–2785 (2020).
Gunnarsson, I. et al. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. 56, 1263–1272 (2007).
Reddy, V. R. et al. Disparity in peripheral and renal B-cell depletion with rituximab in systemic lupus erythematosus: an opportunity for obinutuzumab? Rheumatology 61, 2894–2904 (2022).
Nishath, H. et al. Persistence of immunoglobulin-producing cells in parotid salivary glands of patients with primary Sjögren’s syndrome after B cell depletion therapy. Ann. Rheum. Dis. 71, 1881 (2012).
Ramwadhdoebe, T. H. et al. Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology 58, 1075–1085 (2019).
Thurlings, R. M. et al. Clinical response, pharmacokinetics, development of human anti-chimaeric antibodies, and synovial tissue response to rituximab treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 69, 409–412 (2010).
Teng, Y. K., Levarht, E. W., Toes, R. E., Huizinga, T. W. & van Laar, J. M. Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation. Ann. Rheum. Dis. 68, 1011–1016 (2009).
Kavanaugh, A. et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann. Rheum. Dis. 67, 402–408 (2008).
Hassan, S. U., Md Yusof, M. Y., Emery, P., Dass, S. & Vital, E. M. Biologic sequencing in systemic lupus erythematosus: after secondary non-response to rituximab, switching to humanised anti-CD20 agent is more effective than belimumab. Front. Med. 7, 498 (2020).
Vital, E. M. et al. Brief report: responses to rituximab suggest B cell-independent inflammation in cutaneous systemic lupus erythematosus. Arthritis Rheumatol. 67, 1586–1591 (2015).
Bao, A., Petri, M. A., Fava, A. & Kang, J. Case series of anifrolumab for treatment of cutaneous lupus erythematosus and lupus-related mucocutaneous manifestations in patients with SLE. Lupus Sci. Med. 10, e001007 (2023).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
He, J. & Li, Z. Dilemma of immunosuppression and infection risk in systemic lupus erythematosus. Rheumatology 62, i22–i29 (2023).
Rodziewicz, M. et al. Early infection risk in patients with systemic lupus erythematosus treated with rituximab or belimumab from the British Isles Lupus Assessment Group Biologics Register (BILA-BR): a prospective longitudinal study. Lancet Rheumatol. 5, e284–e292 (2023).
Furie, R. A. et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 81, 100–107 (2022).
Migita, K. et al. Glucocorticoid therapy and the risk of infection in patients with newly diagnosed autoimmune disease. Medicine 92, 285–293 (2013).
Patel, N. J. et al. Coronavirus disease 2019 outcomes among recipients of anti-CD20 monoclonal antibodies for immune-mediated diseases: a comparative cohort study. ACR Open. Rheumatol. 4, 238–246 (2022).
Md Yusof, M. Y. et al. Breakthrough SARS-CoV-2 infections and prediction of moderate-to-severe outcomes during rituximab therapy in patients with rheumatic and musculoskeletal diseases in the UK: a single-centre cohort study. Lancet Rheumatol. 5, e88–e98 (2023).
Kawano, Y. et al. Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: from the first wave through the initial Omicron wave. Ann. Rheum. Dis. 81, 1742–1749 (2022).
Md Yusof, M. Y. et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. 71, 1812–1823 (2019).
Fassbinder, T. et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res. Ther. 17, 92 (2015).
Marco, H. et al. The effect of rituximab therapy on immunoglobulin levels in patients with multisystem autoimmune disease. BMC Musculoskelet. Disord. 15, 178 (2014).
Masoud, S., McAdoo, S. P., Bedi, R., Cairns, T. D. & Lightstone, L. Ofatumumab for B cell depletion in patients with systemic lupus erythematosus who are allergic to rituximab. Rheumatology 57, 1156–1161 (2018).
Cinar, O. K. et al. Ofatumumab use in juvenile systemic lupus erythematosus: a single centre experience. Lupus 30, 527–530 (2021).
Haarhaus, M. L., Svenungsson, E. & Gunnarsson, I. Ofatumumab treatment in lupus nephritis patients. Clin. Kidney J. 9, 552–555 (2016).
Mysler, E. F. et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. 65, 2368–2379 (2013).
Niederfellner, G. et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood 118, 358–367 (2011).
Herter, S. et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol. Cancer Ther. 12, 2031–2042 (2013).
Tipton, T. R. W. et al. Antigenic modulation limits the effector cell mechanisms employed by type I anti-CD20 monoclonal antibodies. Blood 125, 1901–1909 (2015).
Reddy, V. et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 56, 1227–1237 (2017).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT02550652 (2024).
Rovin, B. H. et al. Kidney outcomes and preservation of kidney function with obinutuzumab in patients with lupus nephritis: a post hoc analysis of the NOBILITY trial. Arthritis Rheumatol. 76, 247–254 (2024).
Genentech. Positive phase III results for Genentech’s Gazyva show superiority to standard therapy alone in people with lupus nephritis. Genentech www.gene.com/media/press-releases/15038/2024-09-25/positive-phase-iii-results-for-genentech (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04221477 (2024).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Müller, F. et al. CD19 CAR T-cell therapy in autoimmune disease – a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Cordas dos Santos, D. M. et al. A systematic review and meta-analysis of nonrelapse mortality after CAR T cell therapy. Nat. Med. 30, 2667–2678 (2024).
Verdun, N. & Marks, P. Secondary cancers after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 390, 584–586 (2024).
Zhang, W. et al. Treatment of systemic lupus erythematosus using BCMA-CD19 compound CAR. Stem Cell Rev. Rep. 17, 2120–2123 (2021).
Doglio, M. et al. Regulatory T cells expressing CD19-targeted chimeric antigen receptor restore homeostasis in systemic lupus erythematosus. Nat. Commun. 15, 2542 (2024).
Lee, J. et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J. Clin. Invest. 130, 6317–6324 (2020).
Tur, C. et al. CD19-CAR T-cell therapy induces deep tissue depletion of B cells. Ann. Rheum. Dis. https://doi.org/10.1136/ard-2024-226142 (2024).
Schett, G., Mackensen, A. & Mougiakakos, D. CAR T-cell therapy in autoimmune diseases. Lancet 402, 2034–2044 (2023).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Wang, X. et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell 187, 4890–4904.e9 (2024).
Klein, C., Brinkmann, U., Reichert, J. M. & Kontermann, R. E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug. Discov. 23, 301–319 (2024).
Gruen, M., Bommert, K. & Bargou, R. C. T-cell-mediated lysis of B cells induced by a CD19xCD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol. Immunother. 53, 625–632 (2004).
Subklewe, M. et al. Application of blinatumomab, a bispecific anti-CD3/CD19 T-cell engager, in treating severe systemic sclerosis: a case study. Eur. J. Cancer 204, 114071 (2024).
Bucci, L. et al. Bispecific T cell engager therapy for refractory rheumatoid arthritis. Nat. Med. 30, 1593–1601 (2024).
Alexander, T., Krönke, J., Cheng, Q., Keller, U. & Krönke, G. Teclistamab-induced remission in refractory systemic lupus erythematosus. N. Engl. J. Med. 391, 864–866 (2024).
Hagen, M. et al. BCMA-targeted T-cell-engager therapy for autoimmune disease. N. Engl. J. Med. 391, 867–869 (2024).
Moreau, P. et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 387, 495–505 (2022).
Parodis, I. et al. Attainment of remission and low disease activity after treatment with belimumab in patients with systemic lupus erythematosus: a post-hoc analysis of pooled data from five randomised clinical trials. Lancet Rheumatol. 6, e751–e761 (2024).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Vincent, F. B., Morand, E. F., Schneider, P. & Mackay, F. The BAFF/APRIL system in SLE pathogenesis. Nat. Rev. Rheumatol. 10, 365–373 (2014).
Wu, D. et al. Telitacicept in patients with active systemic lupus erythematosus: results of a phase 2b, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 83, 475 (2024).
Merrill, J. T. et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. 70, 266–276 (2018).
Isenberg, D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann. Rheum. Dis. 74, 2006–2015 (2015).
Carter, L. M., Isenberg, D. A. & Ehrenstein, M. R. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. 65, 2672–2679 (2013).
Cambridge, G. et al. B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann. Rheum. Dis. 67, 1011–1016 (2008).
Vallerskog, T. et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 8, R167 (2006).
Thien, M. et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785–798 (2004).
Hsu, B. L., Harless, S. M., Lindsley, R. C., Hilbert, D. M. & Cancro, M. P. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J. Immunol. 168, 5993–5996 (2002).
Parameswaran, R. et al. Effector-mediated eradication of precursor B acute lymphoblastic leukemia with a novel Fc-engineered monoclonal antibody targeting the BAFF-R. Mol. Cancer Ther. 13, 1567–1577 (2014).
McWilliams, E. M. et al. Anti-BAFF-R antibody VAY-736 demonstrates promising preclinical activity in CLL and enhances effectiveness of ibrutinib. Blood Adv. 3, 447–460 (2019).
Bowman, S. J. et al. Safety and efficacy of subcutaneous ianalumab (VAY736) in patients with primary Sjögren’s syndrome: a randomised, double-blind, placebo-controlled, phase 2b dose-finding trial. Lancet 399, 161–171 (2022).
Lee, S.-S. et al. Interim safety and efficacy of subcutaneous (s.c.) dose ianalumab (VAY736; anti-BAFF-R mAb) administered monthly over 28 weeks in patients with systemic lupus erythematosus (SLE) [abstract LO-021]. Lupus Sci. Med. 10 (Suppl. 1), A17–A18 (2023).
Cortés-Hernández, J. et al. Safety and efficacy of subcutaneous (s.c.) dose ianalumab (VAY736; anti-BAFFR mAb) administered monthly over 28 weeks in patients with systemic lupus erythematosus (SLE) [abstract POS0120]. Ann. Rheum. Dis. 82, 275–276 (2023).
Atisha-Fregoso, Y. et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol. 73, 121–131 (2021).
Kraaij, T. et al. Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol. Dial. Transpl. 36, 1474–1483 (2021).
Aranow, C. et al. Efficacy and safety of sequential therapy with subcutaneous belimumab and one cycle of rituximab in patients with systemic lupus erythematosus: the phase 3, randomised, placebo-controlled BLISS-BELIEVE study. Ann. Rheum. Dis. 83, 1502–1512 (2024).
Shipa, M. et al. Effectiveness of belimumab after rituximab in systemic lupus erythematosus: a randomized controlled trial. Ann. Intern. Med. 174, 1647–1657 (2021).
van Schaik, M. et al. Efficacy of belimumab combined with rituximab in severe systemic lupus erythematosus: study protocol for the phase 3, multicenter, randomized, open-label Synbiose 2 trial. Trials 23, 939 (2022).
Shipa, M. et al. Identification of biomarkers to stratify response to B-cell-targeted therapies in systemic lupus erythematosus: an exploratory analysis of a randomised controlled trial. Lancet Rheumatol. 5, e24–e35 (2023).
Chen, W. et al. Distinct transcriptomes and autocrine cytokines underpin maturation and survival of antibody-secreting cells in systemic lupus erythematosus. Nat. Commun. 15, 1899 (2024).
Cambridge, G. et al. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheumatol. 54, 3612–3622 (2006).
Ostendorf, L. et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N. Engl. J. Med. 383, 1149–1155 (2020).
Alexander, T. et al. Sustained responses after anti-CD38 treatment with daratumumab in two patients with refractory systemic lupus erythematosus. Ann. Rheum. Dis. 82, 1497–1499 (2023).
Roccatello, D. et al. Daratumumab monotherapy for refractory lupus nephritis. Nat. Med. 29, 2041–2047 (2023).
Holzer, M. T. et al. Daratumumab for autoimmune diseases: a systematic review. RMD Open 9, e003604 (2023).
Obeng, E. A. et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107, 4907–4916 (2006).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Segarra, A. et al. Efficacy and safety of bortezomib in refractory lupus nephritis: a single-center experience. Lupus 29, 118–125 (2020).
Zhang, H. et al. The short-term efficacy of bortezomib combined with glucocorticoids for the treatment of refractory lupus nephritis. Lupus 26, 952–958 (2017).
Walhelm, T. et al. Clinical experience of proteasome inhibitor bortezomib regarding efficacy and safety in severe systemic lupus erythematosus: a nationwide study. Front. Immunol. 12, 756941 (2021).
Ishii, T. et al. Multicenter double-blind randomized controlled trial to evaluate the effectiveness and safety of bortezomib as a treatment for refractory systemic lupus erythematosus. Mod. Rheumatol. 28, 986–992 (2018).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Shao, W. H. & Cohen, P. L. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res. Ther. 13, 202 (2011).
Grieves, J. L. et al. Exonuclease TREX1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease. Proc. Natl Acad. Sci. USA 112, 5117–5122 (2015).
Lee-Kirsch, M. A. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Vinuesa, C. G., Shen, N. & Ware, T. Genetics of SLE: mechanistic insights from monogenic disease and disease-associated variants. Nat. Rev. Nephrol. 19, 558–572 (2023).
Carter, L. M. et al. Blood RNA-sequencing across the continuum of ANA-positive autoimmunity reveals insights into initiating immunopathology. Ann. Rheum. Dis. 83, 1322–1334 (2024).
Care, M. A. et al. Network analysis identifies proinflammatory plasma cell polarization for secretion of ISG15 in human autoimmunity. J. Immunol. 197, 1447–1459 (2016).
Md Yuzaiful Md, Y. et al. Prediction of autoimmune connective tissue disease in an at-risk cohort: prognostic value of a novel two-score system for interferon status. Ann. Rheum. Dis. 77, 1432 (2018).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Lood, C., Arve, S., Ledbetter, J. & Elkon, K. B. TLR7/8 activation in neutrophils impairs immune complex phagocytosis through shedding of FcgRIIA. J. Exp. Med. 214, 2103–2119 (2017).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Gkirtzimanaki, K. et al. IFNα impairs autophagic degradation of mtDNA promoting autoreactivity of SLE monocytes in a STING-dependent fashion. Cell Rep. 25, 921–933.e5 (2018).
Kalaaji, M. et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 71, 664–672 (2007).
DeGiorgio, L. A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001).
Yurasov, S. et al. Persistent expression of autoantibodies in SLE patients in remission. J. Exp. Med. 203, 2255–2261 (2006).
Psarras, A. et al. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat. Commun. 11, 6149 (2020).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Mathian, A. et al. Ultrasensitive serum interferon-α quantification during SLE remission identifies patients at risk for relapse. Ann. Rheum. Dis. 78, 1669–1676 (2019).
Stockfelt, M. et al. Plasma interferon-alpha protein levels during pregnancy are associated with lower birth weight in systemic lupus erythematosus. Rheumatology https://doi.org/10.1093/rheumatology/keae332 (2024).
Laurent, A. et al. Burden of systemic lupus erythematosus in clinical practice: baseline data from the SLE Prospective Observational Cohort Study (SPOCS) by interferon gene signature. Lupus Sci. Med. 10, e001032 (2023).
Castellano, G. et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther. 17, 72 (2015).
Toukap, A. N. et al. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 56, 1579–1588 (2007).
Stockfelt, M. et al. Activated low-density granulocytes in peripheral and intervillous blood and neutrophil inflammation in placentas from SLE pregnancies. Lupus Sci. Med 8, e000463 (2021).
Reynolds, J. A. et al. Type I interferon in patients with systemic autoimmune rheumatic disease is associated with haematological abnormalities and specific autoantibody profiles. Arthritis Res. Ther. 21, 147 (2019).
Torell, A. et al. Low CD4+ T cell count is related to specific anti-nuclear antibodies, IFNα protein positivity and disease activity in systemic lupus erythematosus pregnancy. Arthritis Res. Ther. 26, 65 (2024).
Stockfelt, M. et al. Plasma interferon-alpha is associated with double-positivity for autoantibodies but is not a predictor of remission in early rheumatoid arthritis – a spin-off study of the NORD-STAR randomized clinical trial. Arthritis Res. Ther. 23, 189 (2021).
Bekeredjian-Ding, I. B. et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 174, 4043–4050 (2005).
Jego, G. et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19, 225–234 (2003).
Ittah, M. et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren’s syndrome. Arthritis Res. Ther. 8, R51 (2006).
Eloranta, M. L. et al. Regulation of the interferon-α production induced by RNA-containing immune complexes in plasmacytoid dendritic cells. Arthritis Rheum. 60, 2418–2427 (2009).
Hua, J., Kirou, K., Lee, C. & Crow, M. K. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 54, 1906–1916 (2006).
Chasset, F. et al. Rare diseases that mimic systemic lupus erythematosus (lupus mimickers). Joint Bone Spine 86, 165–171 (2019).
König, N. et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 76, 468–472 (2017).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Crawford, J. D. et al. The XIST lncRNA is a sex-specific reservoir of TLR7 ligands in SLE. JCI Insight 8, e169344 (2023).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. M.S. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.S. declares no competing interests. E.M.V. has received consultancy fees from Roche, GSK, AstraZeneca, UCB, Otsuka, BMS, Pfizer, Abbvie, Pfizer, Alpine, Alumis, Merck, BMS, Aurinia Pharmaceuticals, Lilly and Novartis, and has also received research grants paid to his employer from AstraZeneca and Sandoz. Y.K.O.T. has received grants/research support from the Dutch Arthritis Foundation, Autoimmune Research & Collaboration (ARCH) Foundation, Dutch Kidney Foundation, Netherlands Organization for Scientific Research, GSK, CSL Vifor and LUMC, and has received consulting fees from AstraZeneca, Alexion, GSK, Novartis, Otsuka Pharmaceuticals and Vifor Pharma.
Peer review
Peer review information
Nature Reviews Rheumatology thanks William Stohl, Gregg Silverman and Muhammad Shipa for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- B cell-activating factor
-
Also called B lymphocyte stimulator; a potent B cell activator and survival factor that promotes B cell maturation.
- Double-negative B cells
-
B cells that have class switched and lack expression of IgD but also the memory marker CD27. Of these, DN2 cells have higher expression of CD11c and T-BET and are increased in the circulation in patients with SLE.
- Fcγ receptor III
-
Activating Fc receptor that mediates interaction between the Fc ___domain of antibodies and FcγR-bearing effector cells.
- Plasmablasts
-
A heterogeneous subset of short-lived circulating antibody-producing cells that might lie outside a CD19+ lymphocyte gate in flow cytometry and can be defined as CD3−CD14−CD19+/−CD38++CD27++ mononuclear cells.
- Transitional B cells
-
B cells that have successfully recombined their surface receptor and exited the bone marrow but are not yet fully mature. Depending on their stage of transition, they can be CD24hiCD38hi.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stockfelt, M., Teng, Y.K.O. & Vital, E.M. Opportunities and limitations of B cell depletion approaches in SLE. Nat Rev Rheumatol 21, 111–126 (2025). https://doi.org/10.1038/s41584-024-01210-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01210-9