Abstract
Alphaviruses, like many other arthropod-borne viruses, infect vertebrate species and insect vectors separated by hundreds of millions of years of evolutionary history. Entry into evolutionarily divergent host cells can be accomplished by recognition of different cellular receptors in different species, or by binding to receptors that are highly conserved across species. Although multiple alphavirus receptors have been described1,2,3, most are not shared among vertebrate and invertebrate hosts. Here we identify the very low-density lipoprotein receptor (VLDLR) as a receptor for the prototypic alphavirus Semliki forest virus. We show that the E2 and E1 glycoproteins (E2–E1) of Semliki forest virus, eastern equine encephalitis virus and Sindbis virus interact with the ligand-binding domains (LBDs) of VLDLR and apolipoprotein E receptor 2 (ApoER2), two closely related receptors. Ectopic expression of either protein facilitates cellular attachment, and internalization of virus-like particles, a VLDLR LBD–Fc fusion protein or a ligand-binding antagonist block Semliki forest virus E2–E1-mediated infection of human and mouse neurons in culture. The administration of a VLDLR LBD–Fc fusion protein has protective activity against rapidly fatal Semliki forest virus infection in mouse neonates. We further show that invertebrate receptor orthologues from mosquitoes and worms can serve as functional alphavirus receptors. We propose that the ability of some alphaviruses to infect a wide range of hosts is a result of their engagement of evolutionarily conserved lipoprotein receptors and contributes to their pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this study are available within the Article and its Supplementary Information. Confocal microscopy images that support the findings of this study are available at https://omero.hms.harvard.edu/webclient/?show=project-8752. Any other relevant data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Custom pipelines built in Arivis 4DFusion 3.4 analysis software used for this study are available at https://github.com/paulamonterollopis/Viral_Particle_on_Cells_Arivis.
References
Rose, P. P. et al. Natural resistance-associated macrophage protein is a cellular receptor for Sindbis virus in both insect and mammalian hosts. Cell Host Microbe 10, 97–104 (2011).
Zhang, R. et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 557, 570–574 (2018).
Ma, H. et al. LDLRAD3 is a receptor for Venezuelan equine encephalitis virus. Nature 588, 308–314 (2020).
Cheng, R. H. et al. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 80, 621–630 (1995).
Paredes, A. M. et al. Three-dimensional structure of a membrane-containing virus. Proc. Natl Acad. Sci. USA 90, 9095–9099 (1993).
Paredes, A. M., Simon, M. N. & Brown, D. T. The mass of the Sindbis virus nucleocapsid suggests it has T = 4 icosahedral symmetry. Virology 187, 329–332 (1992).
Kuhn, R. J., Niesters, H. G., Hong, Z. & Strauss, J. H. Infectious RNA transcripts from Ross River virus cDNA clones and the construction and characterization of defined chimeras with Sindbis virus. Virology 182, 430–441 (1991).
Nimpf, J. & Schneider, W. J. From cholesterol transport to signal transduction: low density lipoprotein receptor, very low density lipoprotein receptor, and apolipoprotein E receptor-2. Biochim. Biophys. Acta 1529, 287–298 (2000).
Poirier, S., Mamarbachi, M., Chen, W. T., Lee, A. S. & Mayer, G. GRP94 regulates circulating cholesterol levels through blockade of PCSK9-induced LDLR degradation. Cell Rep. 13, 2064–2071 (2015).
Marceau, C. D. et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 (2016).
Zhang, R. et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168 (2016).
Willnow, T. E., Armstrong, S. A., Hammer, R. E. & Herz, J. Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc. Natl Acad. Sci. USA 92, 4537–4541 (1995).
Lozzio, C. B. & Lozzio, B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45, 321–334 (1975).
Dlugosz, P. & Nimpf, J. The reelin receptors apolipoprotein E receptor 2 (ApoER2) and VLDL receptor. Int. J. Mol. Sci. 19, 3090 (2018).
D’Arcangelo, G. et al. Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 (1999).
D’Arcangelo, G. et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723 (1995).
Brandes, C. et al. Avian and murine LR8B and human apolipoprotein E receptor 2: differentially spliced products from corresponding genes. Genomics 42, 185–191 (1997).
Kim, D. H. et al. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 271, 8373–8380 (1996).
Clatworthy, A. E. et al. Expression and alternate splicing of apolipoprotein E receptor 2 in brain. Neuroscience 90, 903–911 (1999).
Lane-Donovan, C. & Herz, J. The ApoE receptors Vldlr and Apoer2 in central nervous system function and disease. J. Lipid Res. 58, 1036–1043 (2017).
Akahata, W. et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 16, 334–338 (2010).
Ko, S. Y. et al. A virus-like particle vaccine prevents equine encephalitis virus infection in nonhuman primates. Sci. Transl. Med. 11, eaav3113 (2019).
Fazakerley, J. K. Semliki forest virus infection of laboratory mice: a model to study the pathogenesis of viral encephalitis. Arch. Virol. Suppl. 2004, 179–190 (2004).
Willems, W. R. et al. Semliki forest virus: cause of a fatal case of human encephalitis. Science 203, 1127–1129 (1979).
Lagomarsino, V. N. et al. Stem cell-derived neurons reflect features of protein networks, neuropathology, and cognitive outcome of their aged human donors. Neuron 109, 3402–3420 e3409 (2021).
Bradish, C. J., Allner, K. & Maber, H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J. Gen. Virol. 12, 141–160 (1971).
Bradish, C. J. & Allner, K. The early responses of mice to respiratory or intraperitoneal infection by defined virulent and avirulent strains of Semliki forest virus. J. Gen. Virol. 15, 205–218 (1972).
Pattyn, S. R., De Vleesschauwer, L. & van der Groen, G. Replication of arboviruses in mouse organ cultures. II. Multiplication of virulent and avirulent Semliki Forest and western equine encephalitis viruses in mouse organ cultures. Arch. Virol. 49, 33–37 (1975).
Fleming, P. Age-dependent and strain-related differences of virulence of Semliki Forest virus in mice. J. Gen. Virol. 37, 93–105 (1977).
Woodward, C. G., Marshall, I. D. & Smith, H. Investigations of reasons for the avirulence of the A7 strain of Semliki Forest virus in adult mice. Br. J. Exp. Pathol. 58, 616–624 (1977).
Trommsdorff, M. et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 (1999).
Van Hoof, D., Rodenburg, K. W. & Van der Horst, D. J. Insect lipoprotein follows a transferrin-like recycling pathway that is mediated by the insect LDL receptor homologue. J. Cell Sci. 115, 4001–4012 (2002).
Van der Horst, D. J., Roosendaal, S. D. & Rodenburg, K. W. Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol. Cell. Biochem. 326, 105–119 (2009).
Finkelshtein, D., Werman, A., Novick, D., Barak, S. & Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl Acad. Sci. USA 110, 7306–7311 (2013).
Ashrafi, K. et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421, 268–272 (2003).
Dieckmann, M., Dietrich, M. F. & Herz, J. Lipoprotein receptors-an evolutionarily ancient multifunctional receptor family. Biol. Chem. 391, 1341–1363 (2010).
Atkins, G. J., Sheahan, B. J. & Mooney, D. A. Pathogenicity of Semliki Forest virus for the rat central nervous system and primary rat neural cell cultures: possible implications for the pathogenesis of multiple sclerosis. Neuropathol. Appl. Neurobiol. 16, 57–68 (1990).
Johnson, R. T., McFarland, H. F. & Levy, S. E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J. Infect. Dis. 125, 257–262 (1972).
Hofer, F. et al. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl Acad. Sci. USA 91, 1839–1842 (1994).
Marlovits, T. C., Abrahamsberg, C. & Blaas, D. Very-low-density lipoprotein receptor fragment shed from HeLa cells inhibits human rhinovirus infection. J. Virol. 72, 10246–10250 (1998).
Bates, P., Young, J. A. & Varmus, H. E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74, 1043–1051 (1993).
Yamamoto, S. et al. Lipoprotein receptors redundantly participate in entry of Hepatitis C virus. PLoS Pathog. 12, e1005610 (2016).
Ujino, S. et al. Hepatitis C virus utilizes VLDLR as a novel entry pathway. Proc. Natl Acad. Sci. USA 113, 188–193 (2016).
Agnello, V., Abel, G., Elfahal, M., Knight, G. B. & Zhang, Q. X. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl Acad. Sci. USA 96, 12766–12771 (1999).
Ganaie, S. S. et al. Lrp1 is a host entry factor for Rift Valley fever virus. Cell 184, 5163–5178.e5124 (2021).
Tao, L. et al. Sulfated glycosaminoglycans and low-density lipoprotein receptor contribute to Clostridium difficile toxin A entry into cells. Nat. Microbiol. 4, 1760–1769 (2019).
Demogines, A., Abraham, J., Choe, H., Farzan, M. & Sawyer, S. L. Dual host-virus arms races shape an essential housekeeping protein. PLoS Biol. 11, e1001571 (2013).
Gruszczyk, J. et al. Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science 359, 48–55 (2018).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Saul, S. et al. Differences in processing determinants of nonstructural polyprotein and in the sequence of nonstructural protein 3 affect neurovirulence of Semliki Forest virus. J. Virol. 89, 11030–11045 (2015).
Finkbeiner, S. & Stevens, C. F. Applications of quantitative measurements for assessing glutamate neurotoxicity. Proc. Natl Acad. Sci. USA 85, 4071–4074 (1988).
Zhang, Y. et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Maherali, N. et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 3, 340–345 (2008).
Jemielity, S. et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 9, e1003232 (2013).
Bausch-Fluck, D. et al. A mass spectrometric-derived cell surface protein atlas. PLoS ONE 10, e0121314 (2015).
Almen, M. S., Nordstrom, K. J., Fredriksson, R. & Schioth, H. B. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 7, 50 (2009).
da Cunha, J. P. Et al. Bioinformatics construction of the human cell surfaceome. Proc. Natl Acad. Sci. USA 106, 16752–16757 (2009).
Joung, J. et al. Genome-scale CRISPR–Cas9 knockout and transcriptional activation screening. Nat. Protoc. 12, 828–863 (2017).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Doench, J. G. et al. Rational design of highly active sgRNAs for CRISPR–Cas9-mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267 (2014).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Saleh, M. C. et al. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature 458, 346–350 (2009).
Aricescu, A. R., Lu, W. & Jones, E. Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D 62, 1243–1250 (2006).
Erasmus, J. H. et al. Novel insect-specific Eilat virus-based chimeric vaccine candidates provide durable, mono- and multivalent, single-dose protection against lethal alphavirus challenge. J. Virol. 92, e01274-17 (2018).
Peng, J. & Gygi, S. P. Proteomics: the move to mixtures. J. Mass Spectrom. 36, 1083–1091 (2001).
Eng, J. K., McCormack, A. L. & Yates, J. R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 5, 976–989 (1994).
Raaben, M. et al. NRP2 and CD63 are host factors for Lujo virus cell entry. Cell Host Microbe 22, 688–696.e685 (2017).
Petersen, T. N., Brunak, S., von Heijne, G. & Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 (2011).
Radoshitzky, S. R. et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 446, 92–96 (2007).
Bajic, G. et al. Influenza antigen engineering focuses immune responses to a subdominant but broadly protective viral epitope. Cell Host Microbe 25, 827–835.e826 (2019).
Clark, S. A. et al. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell 184, 2605–2617.e2618 (2021).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Acknowledgements
J.A. is a recipient of a William Randolph Hearst Foundation and Brigham and Women’s Hospital Young Investigator in Medicine Award, and a Burroughs Wellcome Fund Career Award for Medical Scientists. This work was also supported by a Harvard Milton Fund Award (J.A.), Vallee Scholar Award (J.A.), NIH grant T32 AI007061 (J.A.), NIH grant R24 AI120942 (S.C.W.), NIH grant T32 GM007753 (A.C., K.G.N. and D.V.N.), NIH grant R01 DK127257 (I.M.C.), Burroughs Wellcome Fund Pathogenesis Award (I.M.C.), and NIH T32 CA009216-40 (C.L.), and in part by a grant to Harvard Medical School from the Howard Hughes Medical Institute through the James H. Gilliam Fellowships for Advanced Study program (L.E.C.). The authors acknowledge the MicRoN (Microscopy Resources on the North Quad) Core at Harvard Medical School and the Molecular Electron Microscopy Core Facility at Harvard Medical School for their support and assistance in this work. Additionally, the authors thank A. Burdyniuk and M. Burdyniuk for support in building custom image analysis pipelines; and R. Tomaino from the Taplin Biological Mass Spectrometry Facility at Harvard Medical School for assistance with mass spectrometry of VLPs and data analysis.
Author information
Authors and Affiliations
Contributions
C.L. designed the sgRNA library and the RVP system and performed the CRISPR–Cas9 genetic screen and initial validation. L.E.C. generated cell lines, RVPs and recombinant proteins, and performed infectivity studies for validation with RVPs with assistance from S.A.C., A.C., K.G.N., D.V.N., H.L. and V.B. S.A.C. produced recombinant proteins, and generated cell lines, SINV chimeras and VLPs, and performed experiments with VLPs and SINV chimeras. S.A.C. additionally performed mass spectrometry experiments, BLI experiments and confocal microscopy experiments, the latter of which were performed with assistance from P.M.L. P.M.L. developed the imaging workflow and analysed confocal microscopy data with S.A.C. S.A.C., A.C., P.Y. and V.B. purified RVPs and VLPs for characterization, and A.C. performed negative-stain electron microscopy with VLPs. J.L., K.S.P. and S.C.W. designed and executed experiments with wild-type, replication-competent viruses including in vitro and in vivo studies. D.V.N. and I.M.C. provided mouse cortical neurons and assisted with RVP infectivity studies of mouse and human cortical neurons. H.L. and T.L.Y.-P. provided human iPS cell-derived neurons. I.S., A.A.A. and F.C. participated in study conceptualization or provided critical reagents. I.M.C., S.C.W. and J.A. acquired funding. J.A. wrote the original draft of the manuscript and all authors participated in reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature thanks Laurie Silva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
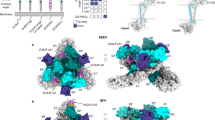
Extended Data Fig. 1 Screening strategy, reporter virus particle system, and gating strategy.
a, Ross River (RRV) reporter virus particle (RVP) system. Cells are transfected with two plasmids. CD20 or GFP is included as a reporter downstream of the capsid (C) after a 2A peptide sequence. The arrow indicates a subgenomic promoter. b, SDS-PAGE gel of purified RVPs imaged with a stain-free imaging system. The experiment was performed twice independently, and a representative gel image is shown. c, Screening strategy. HEK 293T-Cas9 cells are first transduced with the guide (sgRNA) library using vesicular stomatitis virus (VSV) glycoprotein G pseudotyped lentiviruses and are then infected with RVPs expressing CD20. Infected cells are depleted using magnetic beads against CD20. Selection is repeated iteratively to improve the signal-to-noise ratio of the screen. Non-infected, CD20 negative cells are sequenced using next generation sequencing at the final step. See Methods for additional details. d, Coomassie-stained SDS-PAGE gel of purified virus-like particles (VLPs). The experiment was performed twice independently, and a representative gel image is shown. e, Flow cytometry gating strategy for quantification of GFP-expressing cells after RVP infection. K562 cells expressing human VLDLR (top panels) or wild-type (WT) K562 cells (bottom panels) were infected with GFP-expressing SFV RVPs. The percentage of cells falling within each gate is shown. The example is from an experiment shown in Fig. 4e. f, Flow cytometry gating strategy for detection of receptor cell surface staining. K562 cells overexpressing VLDLR (top panels) or WT K562 cells (bottom panels) were stained with RAPFLAG and a FLAG-APC antibody was used for detection. In the rightmost panel, the staining of each cell type is overlaid to allow for comparison. The example is from an experiment shown in Extended Data Fig. 3c. M: molecular weight marker. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 Knockout cell line validation and antibody blocking of SFV E2–E1-mediated entry into multiple cell lines.
a, Genotyping DNA gel (left panel) and anti-VLDLR (α-VLDLR) antibody cell surface staining of WT HEK 293T (middle panel) or HEK 293T VLDLR clonal knockout (K.O.) cells (right panel) as monitored by FACS. The experiment was performed at least twice independently, and a representative gel image is shown. b, Anti-VLDLR (α-VLDLR) cell surface staining of WT HEK 293T, HEK 293T VLDLR K.O., and HEK 293T VLDLR K.O. cells transiently transfected with cDNA encoding VLDLR-Flag (VLDLRFLAG) as monitored by FACS. c, α-VLDLR cell surface staining of the indicated cell types as monitored by FACS. d, The indicated cell types were infected with GFP-expressing SFV single-cycle RVPs in the presence or absence of a α-VLDLR or an anti-HLA control antibody (α-HLA) and infection was measured by FACS. Means ± standard deviation from two experiments performed in triplicate (n = 6) are shown. One-way ANOVA with Tukey’s multiple comparisons test, ****P < 0.0001 (d). For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 3 Immunostaining to monitor cell surface receptor expression.
a, Anti-FLAG (α-FLAG) and anti-MXRA8 (α-Mxra8), staining of WT K562 cells or K562 cells expressing the indicated constructs as monitored by FACS. b, Anti-ApoER2 (α-ApoER2) and anti-LDLR (α-LDLR) staining of the indicated cell types as monitored by FACS. c, RAPFLAG staining of WT K562 cells or K562 cells transduced with the indicated constructs as monitored by ɑ-FLAG-tag staining and FACS.
Extended Data Fig. 4 VLDLR and ApoER2 ligand binding domains directly bind alphavirus E2–E1 proteins.
a, Size exclusion chromatography traces of the indicated purified proteins. Insets are SDS-PAGE gels of the peak fraction. Molecular weight markers are indicated. Each experiment was performed at least twice, and representative traces are shown. b, Electron micrographs of negatively stained purified VLPs. Scale bar is 100 nm. The experiment was performed twice, and representative micrographs are shown. c, Sensorgrams for binding of the indicated alphavirus VLPs to tips coated with VLDLRLBD-Fc, ApoER2LBDiso1-Fc, or Mxra8ect-Fc fusion proteins as measured by biolayer interferometry. Fc fusion protein coated sensor-tips surfaces were incubated with RAP or transferrin, or kinetic buffer alone, and VLPs were associated followed by dissociation. The experiment was performed twice and representative results from one experiment are shown.
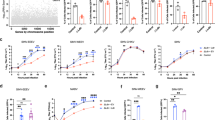
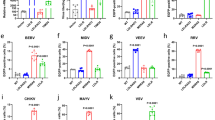
Extended Data Fig. 5 Role of VLDLR and ApoER2 in E2–E1-mediated cellular infection by divergent alphaviruses.
a, Wild-type (WT) or clonal VLDLR knockout (K.O.) HEK 293T cells were infected with GFP-expressing single-cycle alphavirus RVPs with relative infection measured by FACS. EEEV RVPs more efficiently entered VLDLR K.O. cells, which we suspect could be related to clonal variability, as the cell line was generated by clonal dilution. b, Vero cells were infected with GFP-expressing alphavirus single-cycle RVPs in the presence of the indicated antibodies with relative infection measured by FACS. c, Infection of WT or transduced K562 cells with GFP-expressing single-cycle RVPs. Cells were imaged by fluorescence microscopy. Scale bar is 100 μm. The experiment was performed twice, and representative images are shown. d, Infection of WT or transduced K562 cells with GFP-expressing single-cycle RVPs measured by FACS. NRP2 is a control membrane protein. e, K562 cells expressing VLDLR or ApoER2iso2 were infected with the indicated single-cycle RVPs in the presence of RAP, soluble VLDLR LBD (sVLDLRLBD), or a control protein (transferrin, Tf) with infection measured by FACS. f, WT or transduced K562 cells were infected with the indicated GFP-expressing single-cycle RVPs with infection measured by FACS. g, SFV A774 plaque reduction neutralization test with the indicated proteins performed on Vero cells. h, WT K562 cells or K562 cells transduced to express LDLRAD3 were infected with the indicated GFP-expressing single-cycle RVPs with infection measured by FACS. Means ± standard deviation from an experiment performed once in triplicate (n = 3) (a), or experiments performed twice in triplicate (n = 6) with similar results (b, d–h). One-way ANOVA with Tukey’s multiple comparisons test, ****P < 0.0001 (a, b, d–h). Two-way ANOVA with Šídák’s multiple comparison test, ****P < 0.0001 (g). Cell surface expression of constructs used in (c), (d), and (f) was confirmed with immunostaining (see Extended Data Fig. 3).
Extended Data Fig. 6 Ligand-binding ___domain sequence alignment and ___domain organization of ApoER2 constructs.
a, Sequence alignment of the Homo sapiens, Mus musculus, Equus caballus, and Sturnus vulgaris ApoER2 ligand binding domains. The LDLR class A (LA) repeats contained in each protein are shown in parentheses. The ___domain numbering is based on the human sequence shown. b, Schematic representation of the ectodomains of ApoER2 constructs used in this study. In mammals, exon regions encoding LA repeats 4-6 are almost exclusively spliced out, while the predominant avian isoforms retain these repeats14. Panel (a) was generated using ESPrit 3.074.
Extended Data Fig. 7 Representative confocal microscopy images for virus-like particle cell binding and internalization.
K562 cells transduced with human VLDLR, human ApoER2iso2, or human MXRA8 were incubated with fluorescently labeled VLPs at 4 °C or 37 °C and then imaged by live cell confocal microscopy. WGA: wheat germ agglutinin. Scale bar is 10 μm. The experiment was performed twice independently, and representative images are shown.
Extended Data Fig. 8 Workflow diagram of the 3-dimensional quantification of virus-like particle cell surface membrane binding and internalization.
a, 3D analysis of multi-colored stacks (pink, VLPs; green, cell membranes) using Arivis 4DFusion. Two custom-made pipelines were used to detect VLPs and cellular compartments. b, VLPs: left panel shows 3D rendering of VLP stacks, and right panel shows 3D rendering of detected VLPs. c, Cellular compartments: left panel shows 3D rendering of cellular membranes stacks; right, top panel shows 3D rendering of the detected cytoplasms (red) overlayed with an enhanced-membrane filter (white); right, bottom panel shows 3D rendering of the detected membranes (yellow). Objects obtained in each pipeline where combined to quantify the number of VLPs in each cellular compartment. d, Top: single plane representation of the detected objects, showing VLPs in the cytoplasm and the membrane. Bottom: 3D-view of the same cell. Related to Fig. 3c and 3d.
Extended Data Fig. 9 Effects of VLDLRLBD-Fc and RAP on E2–E1-mediated neuron infection and viral replication assays.
a, Infection of human neurons derived from induced pluripotent stem cell (iPSCs) with GFP-expressing SFV single-cycle RVPs in the presence of the indicated proteins. Cells were imaged by fluorescence microscopy. The experiment was performed twice with representative images shown. b, Quantification of single-cycle SFV RVP infection of human iPSC-derived neurons for the experiment shown in (a) using a live cell imaging system (see Methods for additional details) . c, Merged phase contrast and fluorescent microscopy for the experiment with mouse cortical neurons shown in Fig. 4a. Scale bars are 100 μm. Magnification is 20X. d, Merged phase contrast and fluorescent microscopy images for the experiment with human neurons shown in (a). Scale bars are 100 μm. Magnification is 10X. e, Viral replication curve for SFV, EEEV, and SINV strains in transduced K562 cells. Means ± standard deviation from two experiments done in triplicate (n = 6) with one-way ANOVA with Tukey’s multiple comparisons test, ****P < 0.0001 (b). Means ± standard deviation from two experiments done in triplicate (n = 6) with two-way ANOVA with Tukey’s multiple comparisons test, *P = 0.0233, ****P < 0.0001 (e).
Extended Data Fig. 10 Sequence alignment and ___domain organization of VLDLR constructs and summary of observed effects with alphavirus RVPs.
a, Sequence alignment of the Homo sapiens, Mus musculus, Equus caballus, Sturnus vulgaris, Aedes aegypti, Aedes albopictus, and C. elegans VLDLR ortholog ligand binding domains. The LDLR class A (LA) repeats contained in each protein are shown in parentheses. The ___domain numbering is based on the human sequence shown. b, Schematic representation of the ectodomains of VLDLR constructs used in this study. c, Summary of effects observed with GFP-expressing RVP infection of K562 cells transduced to express various VLDLR or ApoER2 orthologs derived from data shown in Extended Data Fig. 5d and Fig. 4e and 4f. +++: RVP infection with greater than 50% GFP positive cells achieved with overexpression. ++: RVP infection with 20–50% GFP positive cells achieved with overexpression. +: RVP infection with 5–20% GFP positive cells achieved with overexpression. +/-: RVP infection with less than 5% GFP positive cells of unclear biological significance. -: no enhancement. Panel (a) was generated using ESPrit 3.074.
Supplementary information
Supplementary Figure 1
Uncropped gels for the indicated Extended Data Figures.
Supplementary Table 1
List of genes encoding membrane-associated proteins targeted by the CRISPR–Cas9 library.
Supplementary Table 2
List of genes and scores from the CRISPR–Cas9 screen after MAGeCK analysis.
Supplementary Table 3
Results of mass spectrometry analysis of purified virus-like particles.
Rights and permissions
About this article
Cite this article
Clark, L.E., Clark, S.A., Lin, C. et al. VLDLR and ApoER2 are receptors for multiple alphaviruses. Nature 602, 475–480 (2022). https://doi.org/10.1038/s41586-021-04326-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04326-0
This article is cited by
-
Temporal transcriptional profiling of host cells infected by a veterinary alphaherpesvirus using nanopore sequencing
Scientific Reports (2025)
-
Genome-wide CRISPR screening identifies LRP1 as an entry factor for SFTSV
Nature Communications (2025)
-
VLDLR mediates Semliki Forest virus neuroinvasion through the blood-cerebrospinal fluid barrier
Nature Communications (2024)
-
Structural basis for VLDLR recognition by eastern equine encephalitis virus
Nature Communications (2024)
-
The low-density lipoprotein receptor promotes infection of multiple encephalitic alphaviruses
Nature Communications (2024)