Abstract
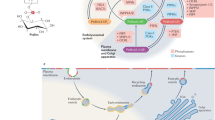
Lysosomal dysfunction has been increasingly linked to disease and normal ageing1,2. Lysosomal membrane permeabilization (LMP), a hallmark of lysosome-related diseases, can be triggered by diverse cellular stressors3. Given the damaging contents of lysosomes, LMP must be rapidly resolved, although the underlying mechanisms are poorly understood. Here, using an unbiased proteomic approach, we show that LMP stimulates a phosphoinositide-initiated membrane tethering and lipid transport (PITT) pathway for rapid lysosomal repair. Upon LMP, phosphatidylinositol-4 kinase type 2α (PI4K2A) accumulates rapidly on damaged lysosomes, generating high levels of the lipid messenger phosphatidylinositol-4-phosphate. Lysosomal phosphatidylinositol-4-phosphate in turn recruits multiple oxysterol-binding protein (OSBP)-related protein (ORP) family members, including ORP9, ORP10, ORP11 and OSBP, to orchestrate extensive new membrane contact sites between damaged lysosomes and the endoplasmic reticulum. The ORPs subsequently catalyse robust endoplasmic reticulum-to-lysosome transfer of phosphatidylserine and cholesterol to support rapid lysosomal repair. Finally, the lipid transfer protein ATG2 is also recruited to damaged lysosomes where its activity is potently stimulated by phosphatidylserine. Independent of macroautophagy, ATG2 mediates rapid membrane repair through direct lysosomal lipid transfer. Together, our findings identify that the PITT pathway maintains lysosomal membrane integrity, with important implications for numerous age-related diseases characterized by impaired lysosomal function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The mass spectrometry data have been deposited to the ProteomeXchange Consortium via the PRIDE55 partner repository with the dataset identifier PXD028852 and 10.6019/PXD028852. Source data are provided with this paper.
References
Pu, J., Guardia, C. M., Keren-Kaplan, T. & Bonifacino, J. S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 129, 4329–4339 (2016).
Platt, F. M., d’Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 4, 27 (2018).
Gómez-Sintes, R., Ledesma, M. D. & Boya, P. Lysosomal cell death mechanisms in aging. Ageing Res. Rev. 32, 150–168 (2016).
Hung, Y.-H., Chen, L. M.-W., Yang, J.-Y. & Yang, W. Y. Spatiotemporally controlled induction of autophagy-mediated lysosome turnover. Nat. Commun. 4, 2111 (2013).
Maejima, I. et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 (2013).
Radulovic, M. et al. ESCRT‐mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 37, e99753 (2018).
Skowyra, M. L., Schlesinger, P. H., Naismith, T. V. & Hanson, P. I. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 360, eaar5078 (2018).
López-Jiménez, A. T. et al. The ESCRT and autophagy machineries cooperate to repair ESX-1-dependent damage at the Mycobacterium-containing vacuole but have opposite impact on containing the infection. PLoS Pathog. 14, e1007501 (2018).
Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801–810 (2012).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Thiele, D. L. & Lipsky, P. E. Mechanism of l-leucyl-l-leucine methyl ester-mediated killing of cytotoxic lymphocytes: dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proc. Natl Acad. Sci. USA 87, 83–87 (1990).
Balla, A. & Balla, T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 16, 351–361 (2006).
Liu, X. & Ridgway, N. D. Characterization of the sterol and phosphatidylinositol 4-phosphate binding properties of Golgi-associated OSBP-related protein 9 (ORP9). PLoS ONE 9, e108368 (2014).
Ngo, M. & Ridgway, N. D. Oxysterol binding protein–related protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol. Biol. Cell 20, 1388–1399 (2009).
Zhou, Y. et al. OSBP-related protein 11 (ORP11) dimerizes with ORP9 and localizes at the Golgi–late endosome interface. Exp. Cell. Res. 316, 3304–3316 (2010).
Levine, T. P. & Munro, S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and-independent components. Curr. Biol. 12, 695–704 (2002).
Miao, G. et al. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev. Cell 56, 427–442.e5 (2021).
Mirza, M. et al. The CLN3 gene and protein: What we know. Mol. Genet. Genomic Med. 7, e859 (2019).
Cheng, X., Shen, D., Samie, M. & Xu, H. Mucolipins: intracellular TRPML1-3 channels. FEBS Lett. 584, 2013–2021 (2010).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783 (2014).
Fujiwara, T., Oda, K., Yokota, S., Takatsuki, A. & Ikehara, Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 263, 18545–18552 (1988).
Baumlova, A. et al. The crystal structure of the phosphatidylinositol 4‐kinase II α. EMBO Rep. 15, 1085–1092 (2014).
Zhou, Q. et al. Molecular insights into the membrane-associated phosphatidylinositol 4-kinase IIα. Nat. Commun. 5, 3552 (2014).
Rost, B. R. et al. Optogenetic acidification of synaptic vesicles and lysosomes. Nat. Neurosci. 18, 1845 (2015).
Gibbons, G. S., Lee, V. M. & Trojanowski, J. Q. Mechanisms of cell-to-cell transmission of pathological tau: a review. JAMA Neurol. 76, 101–108 (2019).
Hammond, G. R. & Balla, T. Polyphosphoinositide binding domains: key to inositol lipid biology. Biochim. Biophys. Acta 1851, 746–758 (2015).
Antonny, B., Bigay, J. & Mesmin, B. The oxysterol-binding protein cycle: burning off PI(4)P to transport cholesterol. Annu. Rev. Biochem. 87, 809–837 (2018).
von Filseck, J. M. et al. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science 349, 432–436 (2015).
Mesmin, B. et al. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 155, 830–843 (2013).
Maeda, K. et al. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature 501, 257–261 (2013).
Chung, J. et al. PI4P/phosphatidylserine countertransport at ORP5-and ORP8-mediated ER–plasma membrane contacts. Science 349, 428–432 (2015).
Kawasaki, A. et al. PI4P/PS countertransport by ORP10 at ER–endosome membrane contact sites regulates endosome fission. J. Cell Biol. 221, e202103141 (2021).
Yeung, T. et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 (2008).
Lim, C.-Y. et al. ER–lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann–Pick type C. Nat. Cell Biol. 21, 1206–1218 (2019).
Subczynski, W. K., Pasenkiewicz-Gierula, M., Widomska, J., Mainali, L. & Raguz, M. High cholesterol/low cholesterol: effects in biological membranes: a review. Cell Biochem. Biophys. 75, 369–385 (2017).
Osawa, T., Ishii, Y. & Noda, N. N. Human ATG2B possesses a lipid transfer activity which is accelerated by negatively charged lipids and WIPI4. Genes Cells 25, 65–70 (2020).
Valverde, D. P. et al. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 218, 1787–1798 (2019).
Osawa, T. et al. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat. Struct. Mol. Biol. 26, 281–288 (2019).
Maeda, S., Otomo, C. & Otomo, T. The autophagic membrane tether ATG2A transfers lipids between membranes. eLife 8, e45777 (2019).
Li, P., Lees, J. A., Lusk, C. P. & Reinisch, K. M. Cryo-EM reconstruction of a VPS13 fragment reveals a long groove to channel lipids between membranes. J. Cell Biol. 219, e202001161 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Varadi, M. et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Giménez-Andrés, M., Čopič, A. & Antonny, B. The many faces of amphipathic helices. Biomolecules 8, 45 (2018).
Drin, G. & Antonny, B. Amphipathic helices and membrane curvature. FEBS Lett. 584, 1840–1847 (2010).
Pranke, I. M. et al. α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J. Cell Biol. 194, 89–103 (2011).
Opaliński, Ł., Kiel, J. A., Williams, C., Veenhuis, M. & Van Der Klei, I. J. Membrane curvature during peroxisome fission requires Pex11. EMBO J. 30, 5–16 (2011).
Dong, R. et al. Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell 166, 408–423 (2016).
Simons, J. P. et al. Loss of phosphatidylinositol 4-kinase 2α activity causes late onset degeneration of spinal cord axons. Proc. Natl Acad. Sci. USA 106, 11535–11539 (2009).
Tan, X., Thapa, N., Sun, Y. & Anderson, R. A. A kinase-independent role for EGF receptor in autophagy initiation. Cell 160, 145–160 (2015).
Hammond, G. R., Machner, M. P. & Balla, T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 205, 113–126 (2014).
Paz, I. et al. Galectin‐3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12, 530–544 (2010).
Xiong, J. et al. Rapid affinity purification of intracellular organelles using a twin strep tag. J. Cell Sci. 132, jcs235390 (2019).
Matyash, V., Liebisch, G., Kurzchalia, T. V., Shevchenko, A. & Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146 (2008).
Gautier, R., Douguet, D., Antonny, B. & Drin, G. HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24, 2101–2102 (2008).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019).
Acknowledgements
We thank members of the Aging Institute at the University of Pittsburgh for helpful discussions and reagents; K. Zhang, G. Shang and M. Hanna for discussions on protein purification and liposome preparation; Z. Wang, H. G. Wang and P. L. Opresko for sharing reagents; MS Bioworks for mass spectrometry services; G. D. Fairn for providing the mCherry-D4H plasmid; V. Olkkonen for the ORP10 plasmid; A. Y. Ting for the TurboID DNA; T. Levine for the OSBP-PH plasmid; G. Du for the LAMP1-GFP-Twin-Strep plasmid; M. Kampmann the eGFP–galectin-3 and Tau.K18(P301L/V337M)-mRuby2 plasmid; C. Rosenmund for the lyso-pHluorin plasmid; T. Balla for the GFP-P4M plasmid; R. Parton for the 2xFYVE_hrs plasmid; S. Grinstein for the Lact-C2 plasmid; C. Tomasetto for the VAPA WT and KD/MD plasmids; and W. Hahn and D. Root for the PI4K2B cDNA. This work was supported by Competitive Medical Research Fund (CMRF) of the University of Pittsburgh Medical Center (UPMC) Health System (J.X.T.) and National Institutes of Health (NIH) grants 1K01AG075142 (J.X.T.), 1R01HL142663 (T.F.), 1R01HL142589 (T.F.), U54AG075931 (T.F.) and P30AG024827 (T.F.). Figs. 1a, 2d, 2j, 3a, 3e and 4d and Extended Data Figs. 5n, 7n and 10 were generated using Biorender.
Author information
Authors and Affiliations
Contributions
J.X.T. designed and performed all experiments, collected and analysed data, designed figures and wrote the manuscript; T.F. supervised the work, provided advice on experiments and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.X.T. declares no competing interests. T.F. is a co-founder and stockholder in Generian Pharmaceuticals.
Peer review
Peer review information
Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Identification of proteins enriched on damaged lysosomes using Lyso-TurboID cells.
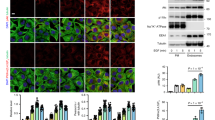
a, Schematic illustration of Lyso-TurboID cells. r1 is the estimated distance between TurboID and the lysosomal membrane; r2 is the estimated radius within which a protein can be biotinylated by TurboID. b, Lyso-TurboID stably expressed in 293T cells colocalizes with the late endosome/lysosome marker CD63. c, Biotin addition enhances biotinylation of proteins that colocalize with Lyso-TurboID. Biotinylated proteins were stained by streptavidin. Note, lysosomal membrane damage by LLOME is known to cause lysosomal enlargement. 293T Lyso-TurboID (green) cells were treated with (500 μM) LLOME for 30 min, followed by another 30 min of biotin treatment (50 μM) without removal of LLOME. Cells were then fixed and permeabilized for streptavidin staining (red). d, Schematic illustration for the purification of proteins recruited to normal and damaged lysosomes. e, Lyso-TurboID is enriched in P20 (pellet after 20,000xg centrifugation) membrane fraction. Samples collected in (d) was analyzed by anti-GFP immunoblotting. Note that some lysosomes shifted from P20 to the heavier P1 fraction in LLOME-treated cells. This is likely because damaged lysosomes are extensively tethered to the ER (See Fig. 2) and thus a fraction of lysosomes might pellet with larger ER fragments in P1. f, Immunoblot analysis confirming the enrichment of mass spectrometry-identified proteins on damaged lysosomes. Protein samples were purified as in (a) from 293T Lyso-TurboID cells. S20, the supernatant above P20, contains the whole cytosol and some light membranes. g, The endosomal sorting complex required for transport (ESCRT) subunits IST1, ALIX, and CHMP3 are all rapidly recruited to damaged lysosomes in multiple cell lines. Cells were treated with 1 mM LLOME for 10 min and stained for the indicated endogenous ESCRT subunits and lysosomal marker LAMP1 after fixation and permeabilization. h, The PtdIns4P probe OSBP-PH-GFP is recruited to damaged lysosomes in U2OS cells as shown by the three channel colocalization between OSBP-PH, LAMP1, and IST1. Cells were treated with 1 mM LLOME for 30 min and then fixed for the staining of endogenous LAMP1 and IST1. DAPI stains the nuclei. Bar, 10 μm. Uncropped western blot images are provided in Supplementary Fig. 1.
Extended Data Fig. 2 Selective PtdIns4P production on damaged lysosomes in different cell lines.
a, b, OSBP-PH-GFP is recruited to damaged lysosomes in U2OS cells as shown by its colocalization with the lysosomal markers LAMP2 (a) and CD63 (b) upon lysosome damage. U2OS cells stably expressing OSBP-PH-GFP were treated with 1 mM LLOME for 30 min and then fixed for immunostaining of the endogenous indicated lysosomal markers. c, OSBP-PH-GFP is recruited to damaged lysosomes in human PC3 prostate adenocarcinoma cells. Cells stably expressing OSBP-PH-GFP were treated with 1 mM LLOME for 30 min and then fixed for immunostaining of endogenous IST1, an ESCRT-III subunit and marker for damaged lysosomes. Pearson’s correlation coefficient of OSBP-PH-GFP and IST1 were quantified; mean ± sem; n = 11 cells from 3 trials for each condition. d, An alternative PtdIns4P probe GFP-P4M is recruited to damaged lysosomes in COS7 cells. Cells were transiently transfected with GFP-P4M. After 24 h, cells were treated with 1 mM LLOME for 30 min and then fixed for immunostaining of endogenous IST1 and LAMP1. Pearson’s correlation coefficient of GFP-P4M and IST1 were quantified; mean ± sem; n = 9 (− LLOME) and 24 (+ LLOME) cells over 3 trials. e, f, Probes for PI(4,5)P2 (e) or PI3P (f) are not recruited to damaged lysosomes. U2OS cells stably expressing the indicated probe were treated with 1 mM LLOME for indicated time periods and then fixed for immunostaining of LAMP1 or IST1. g, Schematic illustration of the dispensability for an intact Golgi in the recruitment of OSBP-PH-GFP to damaged lysosomes. h, Disassembly of the Golgi complex by Brefeldin A does not affect the recruitment of the PtdIns4P probe OSBP-PH-GFP to damaged lysosomes. Cells were pretreated or not with 2 μM Brefeldin A for 10 min followed by the addition of 1 mM LLOME for 30 min. Cells were then fixed for the staining of endogenous LAMP1. i, Lysosomal damage by LLOME does not affect the overall morphologies of the cis- (GM130) and trans-Golgi (Golgin-97) complex. U2OS cells were treated as indicated and stained for endogenous markers of the Golgi complex. DAPI stains the nuclei. Bar, 10 μm. Source data for graphs in this Figure are provided.
Extended Data Fig. 3 Lysosomal damage by diverse factors triggers specific lysosomal recruitment of PI4K2A.
a, Antibody verification for PI4KA, PI4KB, and PI4K2A using the indicated knockdown or knockout cells. Asterisk indicates a nonspecific band. b, Immunoblotting shows the specific enrichment of PI4K2A but not PI4KA or PI4KB on damaged lysosomes. Streptavidin blot was used as a loading control. Note, consistent with subcellular redistribution, PI4K2A levels in the S20 fraction were reduced upon lysosome damage. c, Endogenous PI4K2A forms puncta outside of the Golgi complex upon lysosomal damage by LLOME. GM130 is a marker for the cis-Golgi. Cells were treated with 1 mM LLOME for 10 min, followed by co-staining of endogenous GM130 and PI4K2A. d, Endogenous PI4K2A is rapidly recruited to damaged lysosomes. Cells were treated with 1 mM LLOME for 10 min, followed by co-staining of endogenous LAMP1 and PI4K2A. Quantification of Pearson’s correlation coefficient of PI4K2A and LAMP1 is shown on the right. Mean ± sem; n = 27 cells over three trials for each condition. e, LLOME treatment of COS7 cells induces the formation of endogenous PI4K2A puncta that colocalize with IST1, a marker for damaged lysosomes. f, PI4K2B is not recruited to damaged lysosomes as it does not colocalize with IST1. U2OS cells stably expressing Flag-PI4K2B were stimulated with LLOME for 10 min and stained for endogenous IST1. g, The protein level of PI4K2A does not change within one hour of LLOME treatment. Asterisk indicates a nonspecific band, as demonstrated in panel (a) where knockout of PI4K2A only causes the loss of the lower band. h, The recruitment of endogenous PI4K2A and the ESCRT subunits to damaged lysosomes are independent of each other. Cells were transfected with indicated siRNA; 72 h later, cells were treated with 1 mM LLOME for 10 min and then fixed for co-staining of PI4K2A and IST1. See Supplementary Results for additional discussions about different lysosomal repair pathways. i–k, PI4K2A is recruited to lysosomes damaged by the lysosomotropic detergent O-methyl-serine dodecylamine hydrochloride, MSDH (i), the dipeptide glycyl-l-phenylalanine 2-naphthylamide GPN (j), or the transient expression of SARS-COV2-ORF3A (k). See Supplementary Table 2 for details about the mechanisms of lysosomal damage mediated by these factors. Quantification of Pearson’s correlation coefficient of PI4K2A and LAMP1 is shown for each LMP inducer. Mean ± sem; n = 23 (DMSH), 22 (GPN), 13 (SARS-COV2-ORF3A) cells over three trials for each condition. l, PI4K2A is recruited to phagolysosomes damaged by nano-silica (SiO2). Galectin3 is used as a marker for damaged phagolysosomes. The percentage of phagolysosomes positive for either PI4K2A or Galectin3 or both are quantified on the right. A total of 324 phagolysosomes from 35 silica-treated cells were quantified over three experiments. ND, not detected. Note that the vast majority of Galectin3-positive (indication of severe membrane damage) phagolysosomes are positive for PI4K2A and that a small fraction of PI4K2A-positive large vesicles are negative for Galectin3, likely due to small membrane damages not severe enough to allow the entry of Galectin3. m, CRISPR-based CLN3 knockout causes robust expansion of LAMP1-positive lysosomes that recruit PI4K2A. CLN3 knockout cells were CRISPR pools, with two different sgRNAs showing similar LAMP1 accumulation and PI4K2A recruitment. Quantification of Pearson’s correlation coefficient of PI4K2A and LAMP1 is shown on the right. Mean ± sem; n = 30 cells over three trials for each condition. n, ML-SA1 and MK6–83, two structurally distinct chemical agonists of the main lysosomal Ca2+ channel TRPML1, are each sufficient to trigger lysosomal PI4K2A recruitment. Cells treated with 25 μM of either ML-SA1 or MK6–83 for 40 min were fixed for the staining of endogenous PI4K2A and LAMP1. LLOME treatment for 10 min was used as a positive control. The colocalization of PI4K2A and LAMP1 in ML-SA1 treated cells was quantified. Mean ± sem; n = 26 cells over three trials for each condition. See also Supplementary Results. DAPI stains the nuclei. Bar, 10 μm. Uncropped western blot images are provided in Supplementary Fig. 1. Source data for graphs in this Figure are provided.
Extended Data Fig. 4 PI4K2A-meidated lysosomal PtdIns4P signaling is essential for rapid lysosomal repair.
a, PI4K2A is required for PtdIns4P production on damaged lysosomes. Wild type (WT) or two independent PI4K2A-KO cell lines stably expressing the PtdIns4P probe OSBP-PH-GFP were treated with or without LLOME for 30 min before being fixed for immunostaining of endogenous IST1, a marker for damaged lysosomes. The images show the loss of LMP-induced lysosomal recruitment of OSBP-PH-GFP in PI4K2A-KO cells, which is fully rescued by re-expression of WT PI4K2A. Note, some green puncta still remain in PI4K2A-KO cells, corresponding to the Golgi pool of PtdIns4P. These puncta are absent after Brefeldin A treatment. See panel (c) and Fig. 1g. Bar, 10 μm. b, Western blot verification of PI4K2A levels in wild type or genetically modified U2OS cells. Asterisk indicates a nonspecific band. c, PI4K2A recruitment is responsible for PtdIns4P production on damaged lysosomes. OSBP-PH-GFP-expressing U2OS cells with the indicated genetic modifications were pretreated for 10 min with Brefeldin A to disassemble the Golgi, removing background GFP puncta on the Golgi complex. Cells were then treated with or without 1 mM LLOME for 30 min before immunostaining of endogenous IST1. See quantification in Fig. 1g. Bar, 10 μm. d, The Lyso-pHluorin assay reveals the requirement of PI4K2A and its kinase activity in rapid lysosome repair. Schematic illustration of the assay is shown on the left. Lyso-pHluorin was stably expressed in various U2OS cells with indicated genetic modifications. Cells were treated with 1 mM LLOME for 10 min and then gently washed twice with pre-warmed culture media; fluorescent images were taken at the indicated time points. See quantification in Fig. 1h. Bar, 10 μm. e, Quantification of the lysosomal repair half-life of different cell lines using the Lyso-pHluosin assay. Data were derived from the experiment in (d). Mean ± sem; n = 3. f, The EGFP-Galectin3 rapid lysosomal repair assay demonstrates a role for PI4K2A in protecting lysosomes from severe damage. Schematic illustration of the assay is shown on the left. U2OS cells stably expressing EGFP-Galectin3 were treated with 1 mM LLOME for 60 min to continuously damage lysosomes. Fluorescence microscopic images were taken using live cells. Note, brighter Galectin3 puncta indicates larger and greater unrepaired lysosomal membrane pores. See quantification in Fig. 1i. Bar, 10 μm. g, Rapid lysosomal repair in PI4K2A-KO cells is rescued by expressing LAMP1-mCherry-PI4K2B but not its kinase dead mutant. Cells stably expressing indicated proteins were treated as in (f) and EGFP-Galectin3 punctate intensities were quantified. Mean ± sem; n = 3. Bar, 10 μm. h, Knockout of PI4K2A accelerates tau fibril spreading in cell culture. Top, Schematic illustration of the tau fibril spreading assay. Bottom, cells were incubated with sonicated, non-fluorescent tau fibrils. The percentage of cells showing bright tau-K18-mRuby puncta were examined on a daily basis. See quantification in Fig. 1j. Bar, 60 μm. i, Deletion of PI4K2A exacerbates lipofuscin accumulation in CLN3-knockout fibroblasts. Left, BJ human skin fibroblasts were infected with CRISPR lentivirus for the deletion of CLN3 alone or double deletion of PI4K2A and CLN3. CLN3-sg#1 and PI4K2A-sg#2 were used to obtain the knockout pools. Six days after infection, cells were fixed directly for lipofuscin detection. Right, quantification of lipofuscin intensities. 10–20 random cells per condition; Mean ± sem; n = 3. Bar, 10 μm. DAPI stains the nuclei. NS, not significant. Uncropped western blot images are provided in Supplementary Fig. 1. Source data for graphs in this Figure are provided.
Extended Data Fig. 5 PI4K2A-mediated PtdIns4P signaling recruits ORP9, ORP10, and ORP11 to damaged lysosomes.
a, Antibody verification by western blot using CRISPR knockout pools. Asterisk indicates a nonspecific band. b, ORP10 is mainly found in the P20 fraction and absent from the S20 fraction. P20, pellet after 20,000 xg centrifugation. S20, supernatant after 20,000 xg centrifugation. Asterisk indicates nonspecific bands. c, LMP induces new puncta of EGFP-ORP9/11 on damaged lysosomes. EGFP-ORP9 and -ORP11 are recruited to puncta outside of the Golgi complex upon lysosomal damage by LLOME (top). LMP-induced EGFP-ORP9 puncta colocalize with LAMP1 and IST1 (bottom). d, Endogenous ORP9 and stably expressed EGFP-ORP11 completely colocalize when forming puncta in response to lysosomal damage by LLOME. e, EGFP-ORP11 colocalizes with both PI4K2A and LAMP1 upon lysosomal damage. f, ORP9, but not EGFP-ORP3, forms puncta upon lysosomal damage. g, EGFP-ORP5 is not recruited to damaged lysosomes. Note that the ORP5 punctate structures observed here are not random diffuse signals; they are morphologically distinct from the diffuse signal of ORP3 in (f). When the confocal images were captured at different planes of the cell, the ORP5 structures appear dramatically different31. If the focus plane is away from the bottom of the cell, ORP5 appears as punctate structures on the PM with only diffuse signal in the cytosol. However, when the plane is near the bottom of the cell, as is the case here, ORP5 appears as extensive puncta throughout the cells corresponding to ER-plasma membrane contact sites at the bottom. h, i, Knockdown of PI4K2A by RNA interference (RNAi) blocks the recruitment of EGFP-ORP9 (h) and EGFP-ORP11 (i) to damaged lysosomes. U2OS cells stably expressing EGFP-ORP9 or EGFP-ORP11 were transfected with indicated siRNAs. After 72 h, cells were treated or not with 1 mM LLOME for 10 min and fixed for co-staining of endogenous LAMP1 and IST1. See Supplementary Table 4 for siRNA sequences. j–l, The kinase activity of PI4K2A is required for the recruitment of EGFP-ORP9/10/11 to damaged lysosomes. EGFP-ORP9 (j), EGFP-ORP10 (k), or EGFP-ORP11 (l) were stably expressed in wild type (WT) and PI4K2A knockout (PI4K2A-KO) cells, as well as in PI4K2A-KO cells re-expressing WT (KO + WT) or kinase dead PI4K2A (KO + KDAA). Cells were treated or not with 1 mM LLOME for 10 min and fixed for immunostaining of endogenous IST1. Note, in the absence of PI4K2A activity, all cells lost the recruitment of ORP9/10/11 to damaged lysosomes, as exemplified by the quantification of endogenous ORP9/11 in Fig. 2c. m, LMP-induced puncta of endogenous ORP9 and ORP11 are dependent on PI4K2A and its kinase activity. Indicated cell lines were treated with LLOME for 10 min and fixed for co-staining of endogenous ORP9 and ORP11. See Fig. 2c for quantification. n, o, ORP9 binding to PtdIns4P through its PH ___domain and to VAPA/B through its FFAT motif are both necessary for its efficient recruitment/accumulation on damaged lysosomes. n, schematic illustration of ORP9 mutants. o, indicated ORP9 mutants no longer accumulate on damaged lysosomes. Note, despite the presence of intact PH ___domain for PtdIns4P binding, the ORP9-FYAA mutant is no longer efficiently recruited to damaged lysosomes, suggesting that PtdIns4P-binding alone is insufficient for stable ORP9 association with lysosomes. p, The LMP-induced lysosomal recruitment of ORP9/10/11 requires PtdIns4P-binding through their PH ___domain. RE indicates the point mutation of a conserved arginine residue required for PtdIns4P-binding to the PH ___domain of all the three ORPs. U2OS cells stably expressing mCherry-ORP9, ORP10, or ORP11 or their RE mutants were treated with LLOME for 10 min and then fixed for immunostaining of endogenous LAMP1. Note, while all RE mutants lost lysosomal targeting downstream of LMP, ORP9/10-RE but not ORP11-RE can still be localized to the Golgi complex in resting conditions, indicating different mechanisms for their basal Golgi targeting. See Supplementary Results and Supplementary Table 3 for more details regarding the Golgi and lysosomal targeting of the ORPs. DAPI stains the nuclei. Bar, 10 μm. Uncropped western blot images are provided in Supplementary Fig. 1.
Extended Data Fig. 6 The redundancy of ORP9/10/11/OSBP in their recruitment to damaged lysosomes and in the subsequent establishment of extensive ER-lysosome contacts.
a, The recruitment of EGFP-VAPA to damaged lysosomes is dependent on PI4K2A and its kinase activity. Indicated cells stably expressing EGFP-VAPA were treated or not with LLOME for 10 min and fixed for immunostaining of endogenous LAMP1. The percentages of cells showing extensive VAPA-LAMP1 contacts were quantified in Fig. 2e. b, An EGFP-VAPA mutant that no longer binds to FFAT motifs is poorly recruited to ORP9 puncta induced by lysosomal damage. U2OS cells stably expressing EGFP-VAPA WT or the K94D/M96D mutant were treated with LLOME for 10 min and then fixed for immunostaining of endogenous ORP9. c, Left: ORP9 knockout diminishes ORP11 recruitment to damaged lysosomes, but not vice versa. Wild-type (WT), ORP9 knockout (ORP9-KO), and ORP11-KO U2OS cells were treated or not with 1 mM LLOME for 10 min and then fixed for co-staining of endogenous ORP9 and ORP11. Right: ORP9 knockout does not affect ORP10 recruitment to damaged lysosomes. WT and ORP9-KO cells stably expressing EGFP-ORP10 were treated or not with 1 mM LLOME and fixed for staining of endogenous ORP9. d, ORP10 knockout does not affect the recruitment of ORP9 or ORP11 to damaged lysosomes. WT and ORP10-KO cells were treated or not with 1 mM LLOME for 10 min and then fixed for co-staining of endogenous ORP9 and ORP11. e, ORP9/10/11 triple knockout (TKO) cells show rapid and augmented OSBP recruitment to damaged lysosomes. Wild type U2OS cells or three independent ORP-TKO clones were stimulated with 1 mM LLOME for 10 min and then fixed and stained for endogenous OSBP and LAMP1. Note that OSBP recruitment in wild type cells appeared rather weak, which is likely due to competition from ORP9/10/11, the majorly recruited PtdIns4P effectors. This is likely also the reason why the PtdIns4P probe OSBP-PH-GFP was only substantially recruited after 20–30 min of LLOME treatment (Fig. 1c, d, Supplementary Video 1), whereas PI4K2A and ORP9/10/11 recruitment appear saturated within 10 min (Figs. 1e, 2b, Extended Data Figs. 3, 5). See also Supplementary Results. f, Western blot analysis of indicated protein expression in ORP knockout cells. ORP-QKO indicates knockout of all the ORP proteins recruited to damaged lysosomes identified in mass spectrometry, including ORP9, ORP10, ORP11, and OSBP. Asterisk indicates a nonspecific band. g, The recruitment of PI4K2A and the ESCRT subunit IST1 to damaged lysosomes are unaffected in ORP-QKO cells. h, ORP-QKO cells produce higher levels of PtdIns4P on damaged lysosomes compared with wild type cells. Wild-type (WT) and three independent ORP-QKO cell lines stably expressing the PtdIns4P probe OSBP-PH-GFP were pretreated or not with Brefeldin A to disassemble the Golgi complex, followed by 1 mM LLOME treatment for 30 min. The cells were then fixed to stain for endogenous LAMP1. Note, higher basal lysosomal PtdIns4P levels were also detected in ORP-QKO cells, consistent with previous findings that OSBP plays a role in removing endolysosomal PtdIns4P in resting conditions47. i, Quantification of OSBP-PH-GFP/LAMP1 colocalization in WT or ORP-QKO U2OS cells following LLOME treatment. Note, within 2 h a decrease in colocalization was observed in WT but not ORP-QKO cells. Mean ± sem; n = 15 cells over three trials for each condition. j, Reconstituted ORP9 was robustly recruited to damaged lysosomes in ORP9/11-DKO cells, but not in ORP-TKO or ORP-QKO cells, suggesting that lysosomal recruitment of ORP9 requires the presence of either ORP10 or ORP11. k, The recruitment of reconstituted ORP9, ORP10, ORP11, and OSBP to damaged lysosomes in ORP-QKO cells. When re-expressed in ORP-QKO cells, ORP10 alone, but not ORP9 or ORP11, can be recruited to damaged lysosomes. However, when any two of the three proteins were introduced back to the ORP-QKO cells, both proteins were recruited to damaged lysosomes, consistent with the previously reported hetero-dimerization between ORP9 and ORP1115. Different from the other ORPs, re-expressed OSBP alone was robustly recruited to lysosomes upon LLOME treatment. l, Re-expressing ORP proteins in ORP-QKO cells rescues VAPA recruitment to damaged lysosomes. Left: ORP-QKO cells stably expressing EGFP-VAPA and the indicated mCherry-tagged ORP protein(s) were stimulated with LLOME for 10 min and then fixed for confocal microscopy. See Fig. 2g for quantifications. m, Co-immunoprecipitation experiment showing heterodimerization of Flag-ORP9 with either EGFP-ORP10 or -ORP11 but the absence of ORP9 homodimerization. Whole cell lysates from 293T cells stably expressing indicated proteins were subject to immunoprecipitation using indicated antibodies, followed by immunoblotting. n, OSBP forms homodimers shown by co-immunoprecipitation (co-IP) between EGFP-OSBP and Flag-OSBP. The whole cell lysates from 293T cells stably expressing EGFP-OSBP and Flag-OSBP were subjected to immunoprecipitation using control IgG, Flag, or GFP antibodies. The presence of the two OSBP fusion proteins in each IP samples were analyzed. Note that ORP9 does not form homodimers in similar assays in (m). o, Rescuing lysosomal repair by re-expressing ORP proteins in ORP-QKO cells. Cells stably expressing EGFP-Galectin3 and the indicated mCherry-ORPs were treated continuously with 1 mM LLOME for 60 min. Fluorescence images were taken with live cells. Quantification is shown in Fig. 2i. DAPI stains the nuclei. Bar, 10 μm. NS, not significant. Uncropped western blot images are provided in Supplementary Fig. 1. Source data for graphs in this Figure are provided.
Extended Data Fig. 7 ORP9/10/11 transport phosphatidylserine (PS) to damaged lysosomes.
a, Alignment of human ORPs with yeast Osh6 which specifically transports phosphatidylserine (PS) but not sterols. Bold residues are direct PS contact sites of Osh6. The AAA mutants are designed to lose PS-binding, which have been confirmed in this study. b, Coomassie blue staining of purified ORP9 and ORP11. c, FRET-based assay demonstrating higher lipid transport activity by ORP9/11 (50 nM/50 nM = 100 nM in total) in the presence of PtdIns4P in the acceptor liposome. Note that there was a slight increase in NBD fluorescence in the absence of acceptor liposomes (donor only), which is consistent with ORP9/11-mediated NBD-PS extraction from the donor membranes without further delivery to acceptor membranes. d, ORP9/11 monomers and heterodimers show similar PS transport activity in vitro. ORP9/11 (50 nM/50 nM) heterodimers or monomers (100 nM each) were added to donor and acceptor liposome mixtures, and the changes in NBD fluorescence was monitored over time. The acceptor liposomes in all reactions contained 5% PtdIns4P. See also Supplementary Results. e, The PS probe GFP-Lact-C2 is quickly recruited to damaged lysosomes. U2OS cells stably expressing GFP-Lact-C2 were treated with 1 mM LLOME for the indicated time periods and then briefly washed with 0.1% Triton-X100 (detergent wash, see Methods) to remove background PS signals from the cytosol. The cells were then immediately fixed. f, The recruitment of GFP-Lact-C2 to damaged lysosomes is dependent on PI4K2A and its kinase activity, as well as the ORPs enriched on damaged lysosomes. Cells were treated with LLOME and detergent-washed before fixation and immunostaining. The colocalization of GFP-Lact-C2 and LAMP1 was quantified in Fig. 3d. g, ORP9 is recruited to damaged lysosomes earlier than the PS probe GFP-Lact-C2. U2OS Cells stably expressing GFP-Lact-C2 were treated with LLOME and detergent-washed before fixation and immunostaining of endogenous ORP9 and LAMP1. Arrow indicates ORP9 puncta negative for GFP-Lact-C2. h, The colocalization between LAMP1 and ORP9 or GFP-Lact-C2 in (g) was quantified. Data show mean ± sem of Pearson’s correlation coefficient; n = 15 cells over three trials for each condition. i, LMP-induced lysosomal PS transport in ORP-QKO cells can be rescued by the reconstitution of any two of ORP9/10/11. Cells were treated with 1 mM LLOME for 20 min and detergent-washed before being fixed for immunostaining of LAMP1. j, The colocalization of GFP-Lact-C2 and LAMP1 in (i) was quantified. Data show mean ± sem of Pearson’s correlation coefficient; n = 30 cells for each condition. k, ORP-QKO cells stably expressing GFP-Lact-C2 and mCherry-ORP9/11 or their mutants were treated with LLOME for 20 min and detergent-washed before being fixed for immunostaining of LAMP1. See Fig. 3k for quantification. l, The LMP-induced lysosomal recruitment of VAPA in ORP-QKO cells can be strongly rescued by the AAA mutants (loss of PS binding to the lipid transport ___domain) of ORP9/10 or ORP9/11, weakly rescued by ORP10/11 or the HHAA mutants of ORP9/11, and cannot be rescued by the RE mutants of ORP9/11. See Fig. 3e for details of the mutants. ORP-QKO cells stably expressing EGFP-VAPA and the indicated mCherry-ORP proteins were treated with LLOME for 10 min before fixed for confocal microscopy. m, PIP strips assay showing specific binding of purified EGFP-Lact-C2 to PS. n, Schematic illustration for lysosomal purification, lipid extraction, PVDF spotting, and PS detection. Wild type or ORP-QKO cells stably expressing LAMP1-GFP-twin-Strep were treated or not with 1 mM LLOME for 30 min, followed by lysosomal purification using sptreptavidin beads. Lipids were extracted from lysosomes on beads and dropped onto PVDF membrane for PS detection by purified EGFP-Lact-C2. On the right are representative images of the lipid/protein overlay assay for lysosomal PS detection with LAMP2 immunoblots as total membrane input controls. DAPI stains the nuclei. Bar, 10 μm. NS, not significant. Uncropped western blot images are provided in Supplementary Fig. 1. Source data for graphs in this Figure are provided.
Extended Data Fig. 8 In parallel with ORP9/10/11-mediated PS transport, OSBP drives lysosomal cholesterol transfer as an auxiliary mechanism for rapid lysosomal repair.
a, Rapid lysosomal repair in ORP-QKO cells was only rescued by re-expressing wild type ORP9/11 but not their mutants defective in PS-transport. Cells stably expressing EGFP-Galectin3 and indicated mCherry-ORPs were treated with 1 mM LLOME for 60 min to continuously damage lysosomes. Images were taken using live cells. See image quantification in Fig. 3h. b, Reconstitution of OSBP alone in ORP-QKO cells fully rescues ER tethering to damaged lysosomes. ORP-QKO cells stably expressing EGFP-VAPA and the indicated mCherry-OSBP proteins were treated with LLOME for 10 min before being fixed for confocal microscopy. Similar to ORP mutations in Extended Data Fig. 7k, l, OSBP-2RE loses lysosomal recruitment; OSBP-HHAA does not perform PtdIns4P/cholesterol exchanges between membranes, despite its strong lysosomal recruitment. c, OSBP chimeric proteins rescues ER tethering to damaged lysosomes in ORP-QKO cells. See Fig. 3i for the details of the chimeric proteins. OSBP-Kes1 is constructed similarly to OSBP-Osh6. Cells stably expressing EGFP-VAPA and mCherry-OSBP-X chimeric proteins were stimulated with LLOME for 10 min and then fixed for the staining of endogenous LAMP1. Note, all cells expressing mCherry chimeric proteins showed extensive EGFP-VAPA recruitment to lysosomes, with three-channel colocalization. d, Rapid lysosomal repair in ORP-QKO cells was rescued by re-expressing the indicated chimeric proteins but not the OSBP-Osh6-HHAA mutant defective in PtdIns4P/PS counter transport. Cells stably expressing EGFP-Galectin3 and indicated chimeric proteins were treated as in (a). See image quantification in Fig. 3j. e, LLOME triggers PI4K2A- and OSBP-dependent lysosomal transport of cholesterol. U2OS cells with indicated genetic modifications were stimulated with LLOME for 60 min and then fixed for the staining of endogenous IST1. KDAA is a kinase dead mutant of PI4K2A. 2RE is a mutant of OSBP that is no longer recruited to damaged lysosomes due to loss of PtdIns4P binding to its PH ___domain. f, The colocalization of the cholesterol probe GFP-D4H and IST1 in (e) was quantified. Data show mean ± sem of Pearson’s correlation coefficient; n = 25 cells over 3 trials for each condition. Note that the PS transporters ORP9/11 cannot rescue lysosomal cholesterol accumulation. g, Reconstitution of wild type OSBP, but not its cholesterol transport defective mutants, appears to rescue rapid lysosomal repair in ORP-QKO cells. The same OSBP mutants in (e) and (f) were tested here. Cells stably expressing EGFP-Galectin3 and mCherry-OSBP were continuously treated with 1 mM LLOME and the EGFP-Galectin3 puncta in live cells were analyzed in the right panel. About 50–100 random cells were quantified for each condition. Mean ± sem; n = 3. h, Similar to OSBP-Osh6 in panel c, d, OSBP-Kes1 also rescues rapid lysosomal repair, whereas the cholesterol transport defective HHAA mutant does not. Left, cells stably expressing EGFP-Galectin3 and mCherry-OSBP-Kes1 were continuously treated with 1 mM LLOME and the EGFP-Galectin3 puncta were analyzed with live cells. Right, quantification of the Galectin3 intensities above threshold. About 50–100 random cells were quantified for each condition. Mean ± sem; n = 3. DAPI stains the nuclei. Bar, 10 μm. NS, not significant. Source data for graphs in this Figure are provided.
Extended Data Fig. 9 Independent of macroautophagy, ATG2 mediates rapid lysosomal repair through its lipid transport activity stimulated by PS.
a, Immunoblotting of ATG2A/B protein levels in wild type or ATG2A/B-DKO cells. Two individual clones from each set of CRISPR guides were used for further characterization. b, Double knockout of ATG2A/B causes robust defects of rapid lysosomal repair as shown by the EGFP-Galectin3 assay. The same knockout clones from (a) were used for this assay. Cells were continuously challenged with LLOME and live cell images were captured at indicated time points. Note that re-expression of ATG2A was sufficient to rescue rapid lysosomal repair in the DKO cells. See Fig. 4c for quantifications. c, Immunoblotting of OSBP levels in indicated CRISPR pools. d, Further deletion of OSBP in ATG2A/B-DKO cells causes dramatic defects in rapid lysosomal repair at an early time point. Note that loss of OSBP or ATG2A/B alone does not cause apparent defects within 30 min of LLOME treatment, indicating functional redundancy. e, quantification of the early time point Galectin3 intensities above threshold in (d). About 50–100 random cells were quantified for each condition. Mean ± sem; n = 3. f, Four distinct ATG2A lipid transport mutants are unable to rescue rapid lysosomal repair in ATG2A/B-DKO cells. Stable U2OS cell lines with indicated genetic modifications were continuously challenged with LLOME and live cell images were captured at indicated time points. See quantification in Fig. 4h. g, The ATG2A lipid transport mutants cannot restore autophagic turnover in ATG2A/B-DKO cells. Stable U2OS cell lines with indicated genetic modifications were directly harvested for whole cell lysate extraction followed by immunoblotting of indicated proteins. Data represent more than five experiments. h, EGFP-Galectin3 assay showing the defects of rapid lysosomal repair in wild type cells stably expressing LAMP1-CT but not mCherry-CT or LAMP1-mCherry. See quantification in Fig. 4j. i. Overexpression of different ATG2A-CT fusion proteins including LAMP1-CT does not block macroautophagy. Cells with indicated genetic modifications were treated with 100 nM Bafilomycin A1 for 4 h, followed by immunoblotting of indicated proteins. ATG2A/B-DKO cells served as a positive control for autophagy defects with marked accumulation of both p62 and LC3-II. Quantification of LC3-II intensities normalized to GAPDH is shown. Mean ± sem; n = 3. j, Liposome pull down assays testing the membrane binding capacity of purified MBP–CT or its 5E mutant. k, Highly conserved basic residues in ATG2A-CT. The residues mutated in 5E are in red and also shown in panel (l). l, AlphaFold structure of ATG2A-CT (amino acids 1754–1821). Six highly conserved basic residues are labeled, five of which were mutated in the 5E mutant. m, ATG2A-ΔCT and −5E mutants form dramatically reduced numbers of puncta in response to lysosomal damage. U2OS cells stably expressing EGFP-tagged ATG2A-WT, -ΔCT or −5E were stimulated with LLOME and the numbers of EGFP-ATG2A puncta 20 min after stimulation were determined using live cell imaging. Mean ± sem; n = 15 cells over three trials for each condition. n, EGFP-Galectin3 assay showing the failure of ATG2A-5E mutant in rescuing rapid lysosomal repair in ATG2A/B DKO cells. See quantification in Fig. 4m. o, Knockout of PI4K2A or ORPs does not affect LC3 turnover, indicating normal macroautophagy. Indicated cell lines were treated with 100 nM bafilomycin A1 for four hours followed by whole cell lysate harvest for immunoblotting. p, PI4K2A activity does not affect LLOME-induced LC3 lipidation. U2OS cells with indicated genetic modifications were treated with 1 mM LLOME for 15 to 60 min and then whole cell lysates were analyzed for the level of LC3. Asterisk indicates a nonspecific band. q, LLOME-induced LC3 lipidation is independent of PI4K2A, ORPs, ATG2, and ATG13. U2OS cells with indicated genetic modifications were treated with 1 mM LLOME for 30 min and then whole cell lysates were analyzed for the level of LC3. r, Immunoblotting shows the loss of ATG5 and ATG7 in relevant U2OS knockout cells. Asterisk indicates a nonspecific band. Note that ATG5 is conjugated to ATG12 in wild type cells, which shows a higher band compared with unconjugated ATG5 in ATG7-KO cells. s, ATG5-KO and ATG7-KO cells have normal rapid lysosomal repair, without increased EGFP-Galectin 3 puncta after continuous challenging with 1 mM LLOME. Bar, 10 μm. t, Quantification of EGFP-Galectin3 intensities above threshold in (s). 50–100 random cells were quantified for each condition. Mean ± sem; n = 3. u, Immunoblotting shows no evidence of degradation of either LAMP1 or LAMP2 within four hours of LLOME treatment. U2OS cells were treated with 1 mM LLOME for 1 to 4 h and whole cell lysates was then analyzed for levels of the indicated protein. DAPI stains the nuclei. Bar, 10 μm. Uncropped western blot images are provided in Supplementary Fig. 1. Source data for graphs in this Figure are provided.
Extended Data Fig. 10 Summary illustration of the phosphoinositide-initiated membrane tethering and lipid transport (PITT) pathway for rapid lysosomal repair.
(1) LMP-induced Ca2+ release triggers the rapid lysosomal recruitment of PI4K2A that generates high levels of PtdIns4P on damaged lysosomes. (2) Lysosomal PtdIns4P in turn recruits and stimulates ORP9/10/11 to establish extensive ER-lysosome membrane contacts and mediate subsequent ER-to-lysosome phosphatidylserine (PS) transport. (3) Lysosomal accumulation of PS activates the lipid transporter ATG2 which delivers large amounts of lipids to lysosomes for direct membrane repair. (4) Downstream of PtdIns4P signaling and in parallel of ORP9/10/11, OSBP acts as a redundant membrane tether which transports cholesterol (Chol) rather than PS to damaged lysosomes. Due to the intrinsic capability of cholesterol to improve membrane rigidity and stability, lysosomal cholesterol accumulation might directly assist in membrane repair.
Supplementary information
Supplementary Information
This file contains Supplementary Results and Discussion, Supplementary Tables 1–6 and descriptions for Supplementary Videos 1–5.
Supplementary Fig. 1
Uncropped blots.
Supplementary Video 1
The PtdIns4P probe OSBP-PH-GFP is rapidly recruited to damaged lysosomes. U2OS cells stably expressing OSBP-PH-GFP and LAMP1-mCherry were stimulated with 1 mM LLOME and the trafficking of OSBP-PH-GFP was recorded by live cell imaging.
Supplementary Video 2
ORP9 is rapidly recruited to damaged lysosomes. U2OS cells stably expressing EGFP-ORP9 and LAMP1-mCherry were stimulated with 1 mM LLOME and the trafficking of EGFP-ORP9 was recorded by live cell imaging.
Supplementary Video 3
ORP11 is rapidly recruited to damaged lysosomes. U2OS cells stably expressing EGFP-ORP11 and LAMP1-mCherry were stimulated with 1 mM LLOME and the trafficking of EGFP-ORP11 was recorded by live cell imaging.
Supplementary Video 4
EGFP-ATG2A is rapidly and dynamically recruited to damaged lysosomes. U2OS cells stably expressing EGFP-ATG2A and LAMP1-mCherry were stimulated with 1 mM LLOME and the trafficking of EGFP-ATG2A was recorded by live cell imaging.
Supplementary Video 5
ML-SA1, an agonist of the main lysosomal calcium channel TRPML1, does not trigger lysosomal recruitment of ATG2A. U2OS cells stably expressing EGFP-ATG2A and LAMP1-mCherry were stimulated with 25 μM ML-SA1 and the trafficking of EGFP-ATG2A was recorded by live cell imaging..
Source data
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, J.X., Finkel, T. A phosphoinositide signalling pathway mediates rapid lysosomal repair. Nature 609, 815–821 (2022). https://doi.org/10.1038/s41586-022-05164-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05164-4
This article is cited by
-
Lysosomes: guardians and healers within cells- multifaceted perspective and outlook from injury repair to disease treatment
Cancer Cell International (2025)
-
Genetic targets related to aging for the treatment of coronary artery disease
BMC Medical Genomics (2025)
-
S-palmitoylation modulates ATG2-dependent non-vesicular lipid transport during starvation-induced autophagy
The EMBO Journal (2025)
-
CYP51A1 drives resistance to pH-dependent cell death in pancreatic cancer
Nature Communications (2025)
-
Key challenges and recommendations for defining organelle membrane contact sites
Nature Reviews Molecular Cell Biology (2025)