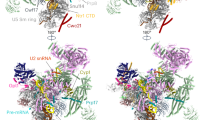
Abstract
Precursor-mRNA (pre-mRNA) splicing requires the assembly, remodelling and disassembly of the multi-megadalton ribonucleoprotein complex called the spliceosome1. Recent studies have shed light on spliceosome assembly and remodelling for catalysis2,3,4,5,6, but the mechanism of disassembly remains unclear. Here we report cryo-electron microscopy structures of nematode and human terminal intron lariat spliceosomes along with biochemical and genetic data. Our results uncover how four disassembly factors and the conserved RNA helicase DHX15 initiate spliceosome disassembly. The disassembly factors probe large inner and outer spliceosome surfaces to detect the release of ligated mRNA. Two of these factors, TFIP11 and C19L1, and three general spliceosome subunits, SYF1, SYF2 and SDE2, then dock and activate DHX15 on the catalytic U6 snRNA to initiate disassembly. U6 therefore controls both the start5 and end of pre-mRNA splicing. Taken together, our results explain the molecular basis of the initiation of canonical spliceosome disassembly and provide a framework to understand general spliceosomal RNA helicase control and the discard of aberrant spliceosomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Three-dimensional cryo-EM composite density maps of the C. elegans ILS′ and ILS″ have been deposited into the EMDB under the accession numbers EMD-19397–EMD-19398. The individual maps 1–27 have been deposited under the accession numbers EMD-50447, EMD-50449–EMD-50569 and EMD-50471–EMD-40475. Three-dimensional cryo-EM composite density maps of the human ILS″ have been deposited in the EMDB under the accession number EMD-19399. The individual human ILS″ maps 1–14 have been deposited under the accession numbers EMD-50477–EMD-50490. The coordinate files of the C. elegans ILS′, C. elegans ILS″, the revised human P complex and the human ILS″ have been deposited into the PDB under the accession numbers 8RO0, 8RO1, 9FMD and 8RO2, respectively.
References
Will, C. L. & Lührmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 3, a003707 (2011).
Bertram, K. et al. Cryo-EM structure of a pre-catalytic human spliceosome primed for activation. Cell 170, 701–713.e11 (2017).
Charenton, C., Wilkinson, M. E. & Nagai, K. Mechanism of 5′ splice site transfer for human spliceosome activation. Science 364, 362–367 (2019).
Tholen, J., Razew, M., Weis, F. & Galej, W. P. Structural basis of branch site recognition by the human spliceosome. Science 375, 50–57 (2022).
Townsend, C. et al. Mechanism of protein-guided folding of the active site U2/U6 RNA during spliceosome activation. Science 370, eabc3753 (2020).
Zhan, X., Yan, C., Zhang, X., Lei, J. & Shi, Y. Structures of the human pre-catalytic spliceosome and its precursor spliceosome. Cell Res. 28, 1129–1140 (2018).
Kastner, B., Will, C. L., Stark, H. & Lührmann, R. Structural insights into nuclear pre-mRNA splicing in higher eukaryotes. Cold Spring Harb. Perspect. Biol. 11, a032417 (2019).
Wan, R., Bai, R., Zhan, X. & Shi, Y. How is precursor messenger RNA spliced by the spliceosome? Annu. Rev. Biochem. 89, 333–358 (2020).
Wilkinson, M. E., Charenton, C. & Nagai, K. RNA splicing by the spliceosome. Annu. Rev. Biochem. 89, 359–388 (2020).
Toroney, R., Nielsen, K. H. & Staley, J. P. Termination of pre-mRNA splicing requires that the ATPase and RNA unwindase Prp43p acts on the catalytic snRNA U6. Genes Dev. 33, 1555–1574 (2019).
Feng, Q., Krick, K., Chu, J. & Burge, C. B. Splicing quality control mediated by DHX15 and its G-patch activator SUGP1. Cell Rep. 42, 113223 (2023).
Fourmann, J.-B., Tauchert, M. J., Ficner, R., Fabrizio, P. & Lührmann, R. Regulation of Prp43-mediated disassembly of spliceosomes by its cofactors Ntr1 and Ntr2. Nucleic Acids Res. 45, 4068–4080 (2017).
Fourmann, J.-B. et al. The target of the DEAH-box NTP triphosphatase Prp43 in Saccharomyces cerevisiae spliceosomes is the U2 snRNP–intron interaction. eLife 5, e15564 (2016).
Koodathingal, P., Novak, T., Piccirilli, J. A. & Staley, J. P. The DEAH box ATPases Prp16 and Prp43 cooperate to proofread 5′ splice site cleavage during pre-mRNA splicing. Mol. Cell 39, 385–395 (2010).
Maul-Newby, H. M. et al. A model for DHX15 mediated disassembly of A-complex spliceosomes. RNA 28, 583–595 (2022).
Garrey, S. M. et al. A homolog of lariat-debranching enzyme modulates turnover of branched RNA. RNA 20, 1337–1348 (2014).
Hang, J., Wan, R., Yan, C. & Shi, Y. Structural basis of pre-mRNA splicing. Science 349, 1191–1198 (2015).
Masaki, S. et al. Identification of the specific interactors of the human lariat RNA debranching enzyme 1 protein. Int. J. Mol. Sci. 16, 3705–3721 (2015).
Yan, C. et al. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science 349, 1182–1191 (2015).
Yoshimoto, R., Okawa, K., Yoshida, M., Ohno, M. & Kataoka, N. Identification of a novel component C2ORF3 in the lariat–intron complex: lack of C2ORF3 interferes with pre-mRNA splicing via intron turnover pathway. Genes Cells 19, 78–87 (2014).
Yoshimoto, R., Kataoka, N., Okawa, K. & Ohno, M. Isolation and characterization of post-splicing lariat–intron complexes. Nucleic Acids Res. 37, 891–902 (2009).
Zhang, X. et al. Structures of the human spliceosomes before and after release of the ligated exon. Cell Res. 29, 274–285 (2019).
Wan, R., Yan, C., Bai, R., Lei, J. & Shi, Y. Structure of an intron lariat spliceosome from Saccharomyces cerevisiae. Cell 171, 120–132.e12 (2017).
Spieth, J. & Lawson, D. in WormBook (ed. The C. elegans Research Community, WormBook) https://doi.org/10.1895/wormbook.1.65.1 (2006).
Chen, W. et al. Endogenous U2·U5·U6 snRNA complexes in S. pombe are intron lariat spliceosomes. RNA 20, 308–320 (2014).
Cao, W., Tran, C., Archer, S. K., Gopal, S. & Pocock, R. Functional recovery of the germ line following splicing collapse. Cell Death Differ. 29, 772–787 (2022).
Ceron, J. et al. Large-scale RNAi screens identify novel genes that interact with the C. elegans retinoblastoma pathway as well as splicing-related components with synMuv B activity. BMC Dev. Biol. 7, 30 (2007).
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003).
Simmer, F. et al. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1, E12 (2003).
Studer, M. K., Ivanović, L., Weber, M. E., Marti, S. & Jonas, S. Structural basis for DEAH-helicase activation by G-patch proteins. Proc. Natl Acad. Sci. USA 117, 7159–7170 (2020).
Tauchert, M. J., Fourmann, J.-B., Lührmann, R. & Ficner, R. Structural insights into the mechanism of the DEAH-box RNA helicase Prp43. eLife 6, e21510 (2017).
Fourmann, J.-B. et al. Dissection of the factor requirements for spliceosome disassembly and the elucidation of its dissociation products using a purified splicing system. Genes Dev. 27, 413–428 (2013).
Small, E. C., Leggett, S. R., Winans, A. A. & Staley, J. P. The EF-G-like GTPase Snu114p regulates spliceosome dynamics mediated by Brr2p, a DExD/H box ATPase. Mol. Cell 23, 389–399 (2006).
Wollenhaupt, J. et al. Intrinsically disordered protein Ntr2 modulates the spliceosomal RNA helicase Brr2. Biophys. J. 114, 788–799 (2018).
Riabov Bassat, D. et al. Structural basis of human U5 snRNP late biogenesis and recycling. Nat. Struct. Mol. Biol. 31, 747–751 (2024).
Schneider, S. et al. Structure of the human 20S U5 snRNP. Nat. Struct. Mol. Biol. 31, 752–756 (2024).
Fica, S. M., Oubridge, C., Wilkinson, M. E., Newman, A. J. & Nagai, K. A human postcatalytic spliceosome structure reveals essential roles of metazoan factors for exon ligation. Science 363, 710–714 (2019).
Bertram, K. et al. Structural insights into the roles of metazoan-specific splicing factors in the human step 1 spliceosome. Mol. Cell 80, 127–139.e6 (2020).
Dybkov, O. et al. Regulation of 3′ splice site selection after step 1 of splicing by spliceosomal C* proteins. Sci. Adv. 9, eadf1785 (2023).
Fica, S. M. et al. RNA catalyses nuclear pre-mRNA splicing. Nature 503, 229–234 (2013).
Zhang, J. et al. DHX15 is involved in SUGP1-mediated RNA missplicing by mutant SF3B1 in cancer. Proc. Natl Acad. Sci. USA 119, e2216712119 (2022).
Han, B. et al. Human DBR1 modulates the recycling of snRNPs to affect alternative RNA splicing and contributes to the suppression of cancer development. Oncogene 36, 5382–5391 (2017).
Didychuk, A. L., Butcher, S. E. & Brow, D. A. The life of U6 small nuclear RNA, from cradle to grave. RNA 24, 437–460 (2018).
Trippe, R. et al. Identification, cloning, and functional analysis of the human U6 snRNA-specific terminal uridylyl transferase. RNA 12, 1494–1504 (2006).
Cartwright-Acar, C. H. et al. A forward genetic screen in C. elegans identifies conserved residues of spliceosomal proteins PRP8 and SNRNP200/BRR2 with a role in maintaining 5′ splice site identity. Nucleic Acids Res. 50, 11834–11857 (2022).
Noble, S. M. & Guthrie, C. Identification of novel genes required for yeast pre-mRNA splicing by means of cold-sensitive mutations. Genetics 143, 67–80 (1996).
Strauss, E. J. & Guthrie, C. A cold-sensitive mRNA splicing mutant is a member of the RNA helicase gene family. Genes Dev. 5, 629–641 (1991).
Koodathingal, P. & Staley, J. P. Splicing fidelity: DEAD/H-box ATPases as molecular clocks. RNA Biol. 10, 1073–1079 (2013).
Bohnsack, K. E., Ficner, R., Bohnsack, M. T. & Jonas, S. Regulation of DEAH-box RNA helicases by G-patch proteins. Biol. Chem. 402, 561–579 (2021).
Plaschka, C., Lin, P.-C. & Nagai, K. Structure of a pre-catalytic spliceosome. Nature 546, 617–621 (2017).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Paix, A., Folkmann, A., Rasoloson, D. & Seydoux, G. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR–Cas9 ribonucleoprotein complexes. Genetics 201, 47–54 (2015).
Mayeda, A. & Krainer, A. R. Preparation of HeLa cell nuclear and cytosolic S100 extracts for in vitro splicing. Methods Mol. Biol. 118, 309–314 (1999).
Kastner, B. et al. GraFix: sample preparation for single-particle electron cryomicroscopy. Nat. Methods 5, 53–55 (2008).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Kimanius, D. et al. Data-driven regularization lowers the size barrier of cryo-EM structure determination. Nat. Methods 21, 1216–1221 (2024).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Varadi, M. et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Kelly, L. A., Mezulis, S., Yates, C., Wass, M. & Sternberg, M. The Phyre2 web portal for protein modelling, prediction, and analysis. Nat. Protoc. 10, 845–858 (2015).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Biesiada, M., Purzycka, K. J., Szachniuk, M., Blazewicz, J. & Adamiak, R. W. Automated RNA 3D structure prediction with RNAComposer. Methods Mol. Biol. 1490, 199–215 (2016).
Trowitzsch, S., Bieniossek, C., Nie, Y., Garzoni, F. & Berger, I. New baculovirus expression tools for recombinant protein complex production. J. Struct. Biol. 172, 45–54 (2010).
Dorfer, V. et al. MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. J. Proteome Res. 13, 3679–3684 (2014).
Käll, L., Canterbury, J. D., Weston, J., Noble, W. S. & MacCoss, M. J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925 (2007).
Taus, T. et al. Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 10, 5354–5362 (2011).
Doblmann, J. et al. apQuant: accurate label-free quantification by quality filtering. J. Proteome Res. 18, 535–541 (2019).
Schwanhäusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Smyth, G. K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article3 (2004).
Tani, H. et al. Real-time monitoring of RNA helicase activity using fluorescence resonance energy transfer in vitro. Biochem. Biophys. Res. Commun. 393, 131–136 (2010).
Yariv, B. et al. Using evolutionary data to make sense of macromolecules with a ‘face-lifted’ ConSurf. Protein Sci. 32, e4582 (2023).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Acknowledgements
We thank members of the Plaschka and Cochella groups for their help and discussions; staff at the Protein Technologies Facility at the Vienna BioCenter Core Facilities (VBCF), a member of the Vienna BioCenter (VBC), for assistance with protein production; staff at the VBCF Electron Microscopy Facility, in particular T. Heuser and H. Kotisch, for support, data collection and maintaining facilities; V.-V. Hodirnau and L. Lovicar at the Institute of Science and Technology Austria EM facility for cryo-EM data collection; R. Zimmermann and his team for computational support; K. Mechtler and E. Roitinger and their team for mass spectrometry; the in-house Molecular Biology Service for reagents; M. Madalinski for peptide synthesis; M. Kostic, C. Bernecky and A. Stark for critical reading of the manuscript. The computational results presented were obtained using the CLIP cluster (https://clip.science). M.K.V. was supported by a Marie Sklodowska-Curie Postdoctoral Fellowship (101022449), P.R. was supported by a Boehringer-Ingelheim Fonds fellowship. L.C. was supported by the National Science Foundation (NSF CAREER Award 2238425). Research in the laboratory of C.P. is supported by Boehringer-Ingelheim, the European Research Council under the Horizon 2020 research and innovation programme (ERC-2020-STG 949081 RNApaxport) and by the Austrian Science Fund (FWF) doc.funds program DOC177-B (RNA@core: Molecular mechanisms in RNA biology).
Author information
Authors and Affiliations
Contributions
M.K.V. designed research, carried out cryo-EM data analyses and structure determination of C. elegans spliceosomes, performed biochemical assays and drafted the initial manuscript. P.R. carried out cryo-EM data analyses and structure determination of the human ILS, aided by M.K.V., and prepared the revised human P complex structure. J.K. generated the 3×Flag–PRP19 C. elegans strains and prepared C. elegans spliceosomes and cryo-EM grids. E.D.C. generated C. elegans syf-2 mutant strains, performed RNAi experiments and analysed the data with L.C. L.V. prepared the human ILS and cryo-EM grids. D.R.-B. generated the human GFP–TFIP11 K562 cell line. M.K.V., P.R. and L.F. purified proteins. A.W.P. grew large GFP–TFIP11 K562 cell cultures and prepared NEs. M.K.V., P.R., L.C. and C.P. analysed data and prepared the manuscript with input from all authors. L.C. designed research, supervised E.D.C. and co-supervised J.K. with C.P. C.P. supervised M.K.V., P.R., L.V., D.R-B., L.F. and A.W.P., designed research and initiated the spliceosome project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Cryo-EM analysis of C. elegans spliceosomes.
a. Schematic of purification of spliceosomes from C. elegans. The endogenous locus of PRP19 was tagged with am N-terminal FLAG-tag using CRISPR/Cas9 and extract was prepared from ~12 million adult worms. After immunopurification (IP) and elution with FLAG peptide, spliceosomes were further purified via a sucrose gradient. b. Coomassie-stained SDS-Poly-Acrylamide Gel (SDS-PAGE) of gradient-purified Ce spliceosomes. This experiment was performed seven times. c. Denoised cryo-EM micrograph of gradient-purified and crosslinked Ce spliceosomes imaged on a Titan Krios with a K3 detector. d. 2D class averages from the dataset. e. Abundance of ILS subunits in gradient-purified sample measured by mass spectrometry. For this analysis we quantified absolute protein abundances by integrating the protein peptide peaks and normalizing to the protein length using iBAQ69, which were then normalized to PRP8. The labels next to the bars indicate how many peptides were identified for each subunit and which percent of the sequence was covered. f. Schematic of the data analysis pipeline. Stringent classification of ~4 million single particle images revealed the ILS′ (~85–90% of ILS particles) and ILS″ (~10–15% of ILS particles, see Supplementary Fig. 1) as the major PRP19-containing spliceosomes populations in Ce extract. Extensive focused refinements of each state yielded a total of 27 maps, revealing the ILS′ and ILS″ in unprecedented detail and facilitating the building of high-quality structural models. For details, see Extended Data Fig. 2 and Supplementary Figs. 1, 2. g. Sequence conservation plot of ILS subunits between human and C. elegans (Ce), and human and Saccharomyces cerevisiae (Sc) shows a highly conserved ILS protein composition between human and Ce.
Extended Data Fig. 2 Comparison of the complete C. elegans ILS″ to a partial human ILS2.
a-b. Side-by-side comparison of the Ce ILS″ cryo-EM density map with the deposited human ILS2 map. Top: Overview, with cryo-EM density colored by subunits. For the human ILS2 (EMD-9647), a low pass filtered map (gaussian filter with a width of three standard deviations) is shown in addition (transparent white surface). Bottom: Zoom-ins to the spliceosomes core reveal nearly indistinguishable densities where high-resolution density is available for both Ce and Hs ILS. c. Coordinate model statistics for Ce ILS″ and Hs ILS2, listing number of residues included as full sidechain models or backbone models, respectively. Numbers in brackets indicate completeness relative to the sum of all residues calculated from deposited sequences for the full-length proteins. d. ILS subunit diagrams indicating which residues are included in coordinate models of the Ce ILS″ or Hs ILS2 as full side chain models (solid fill), backbone models (semi-transparent fill with stripes), or not modelled (transparent fill). Asterisks indicate severe register errors in deposited human ILS2 models in SYF1 (register error of up to 120 residues) and SYF3 (register error of ~20 residues).
Extended Data Fig. 3 Yeast and metazoan ILS architectures are poorly conserved.
a. Comparison of disassembly factors observed in available baker’s yeast (S. cerevisiae, Sc), fission yeast (S. pombe, Sp), human (Hs) or nematode (Ce) ILS structures. b. Cartoon representation of the Ce TFIP11-PAXBP1 heterodimer. c. Cartoon representation of the Sc TFIP11-PAXBP1 homolog Ntr1-Ntr2, with the G-patch factor Ntr1 aligned to its homolog TFIP11. d. Side views of the ILS from Sc, Sp, Hs and Ce, with disassembly factors shown in ribbon representations and the ILS core shown as a transparent white surface. e. Yeast Sc Ntr1-Ntr2 (transparent ribbons) overlayed on the Ce ILS″, revealing substantially different binding sites on the ILS. Structures were aligned on PRP8.
Extended Data Fig. 4 Conformational and compositional changes from ILS′ to ILS″.
a. Close-up view of protein-protein interactions between the PRP8 RNase H (RH) ___domain, TFIP11, PAXBP1 and BRR2 in the ILS′. Protein elements thar are mobile in the ILS′-to-ILS″-transition are shown as ribbons, whereas elements thar are static are shown in addition as transparent surfaces. b. Close-up view of protein-protein interactions between the PRP8 RNase H (RH) ___domain, TFIP11, PAXBP1 and C19L2 in the ILS″. C19L2 binding requires repositioning of the PRP8 RH ___domain and TFIP11–PAXBP1, which displaces the BRR2–PRP8 JAB1/MPN domains from PAXBP1. C19L2 recruits C19L1 by binding its C-terminal CWFJ ___domain. c. Overlay of TFIP11–PAXBP1 in the ILS′ and ILS″ in a 90° rotated view. Yellow arrows connect identical residues in both states. d. Overview of the ILS′. DHX15 (transparent) is likely tethered via the TFIP11 G-patch ___domain but cannot dock onto its target. e. Overview of the ILS″. C19L1 and C19L2 binding allows docking of DHX15, and the associated conformational change in TFIP11–PAXBP1 and PRP8 displaces BRR2. Circled numbers 1 and 2 indicate regions of zoom-ins in panels f, i and j. f. Close-up view of the ILS″ U6 snRNA 3′ end, with DHX15 and the TFIP11 G-patch removed for clarity. The oligo-uridylated and single-stranded U6 snRNA 3′ end is the ideal substrate for DHX15, and SDE2 and SYF2 shield the U2/U6 helix II. g. RNA cryo-EM density in the ILS′. After dissociation of ligated mRNA and catalysis-specific splicing proteins, the RNA active site is more mobile. h. RNA cryo-EM density in the ILS″. Compared to the ILS′, RNA densities are better defined in the ILS″, presumably due to binding of C19L2 (see panel j). i. Continuous cryo-EM density between U2-U6 helix II and the DHX15 active site reveals U6 snRNA as the target for ILS disassembly. j. C19L2 binds the active site RNA network near the branch helix and contacts U2 snRNA, intron-lariat RNA, U5 snRNA, and U6 snRNA.
Extended Data Fig. 5 AlphaFold2 Multimer predictions support disassembly factor interactions.
a. AlphaFold2 Multimer prediction of full-length Ce C19L2–C19L1.The prediction is shown colored by subunit (left) or AlphaFold2 confidence score (per residue local difference distance test, plDDT, right). The prediction supports the binding of the C19L1 CWFJ ___domain to C19L2. The C19L1 MMP ___domain (rendered transparent) is predicted to be collapsed onto the structure, however our experimental cryo-EM density shows that in the ILS″ the C19L1 MMP ___domain is distant from the C19L1 CWFJ–C19L2 complex. Note that the C19L1 CWFJ–C19L2 interaction is predicted with low confidence and in only 2 of 5 models (panel c). b. plDDT scores of the 5 models plotted over the amino acid number. Scores for the 5 models are overlayed. c. Predicted aligned error (PAE) plot of the 5 models, sorted from highest ranked prediction (left) to lowest ranked prediction (right). d. Pull-down experiment with immobilized C19L2(α1-α2) peptide and recombinant C19L1. C19L1 binds the wildtype C19L2(α1-α2) peptide but not a C19L2(α1-α2) scrambled peptide control. Small insets underneath the lanes show fluorescent images of the beads with immobilized fluorescently labelled C19L2 peptides in the fluorescein channel to show equal loading of the wildtype and scrambled C19L2 peptides. This experiment was performed once. e. Overview (left) and close-up (right) view of the Ce C19L1–DHX15–SYF1 interfaces in the ILS″. C19L1 and SYF1 jointly bind a conserved hydrophobic pocket in the DHX15 CTD. The close-up panel shows the DHX15 surface colored by molecular hydrophobicity potential, with white colors indicating hydrophobic surfaces. f. The same close up as in panel e (right), but colored by sequence conservation. Residue conservation scores were obtained from the ConSurf server73. g. AlphaFold2 Multimer prediction of Hs DHX15 with C19L1 and SYF1 suggests a conserved binding mode and conserved hydrophobic residues in the DHX15 CTD, the C19L1 ‘loop 2’, and the SYF1 ‘tether’. h. Pull-down experiment with immobilized SYF1(788−818) peptide and recombinant C19L1 and DHX15. C19L1 and DHX15 both bind the SYF1(788−818) ‘tether’ peptide but not a scrambled peptide control. C19L1 and DHX15 can also simultaneously bind to the wildtype but not the scrambled peptide. Small insets underneath the lanes show images of the beads with immobilized fluorescently labelled SYF1 peptides in the fluorescein channel to show equal loading of the wildtype and scrambled SYF1 peptide. This experiment was performed three times. i. Predicted aligned error plots of the Ce and Hs DHX15–C19L1–SYF1 AlphaFold2 Multimer predictions. j. Predicted local distance difference test (plDDT) plots of the AlphaFold2 Multimer predictions from panel i. k. SYF3 might assist with positioning the C19L1 MMP ___domain in the ILS″. Zoom-in of an AlphaFold2 Multimer prediction between Hs SYF3 and C19L1, highlighting the interface between a SYF3 C-terminal β-hairpin (residues 780–805) that extends the C19L1 MMP ___domain central β-sheet. The SYF3 β-hairpin might be flexible relative to the HAT (half a tetratricopeptide repeat) ___domain through movement around a hinge residue (indicated with an arrow). l. As panel k, but for the Ce proteins. m. The Ce SYF3–C19L1 AlphaFold2 Multimer prediction overlayed onto C19L1 in the Ce ILS″ cryo-EM structure. Cryo-EM density is shown as a transparent surface. Weak density is visible at the position predicted for SYF3 by AlphaFold2 Multimer, and also near the end of the SYF3 HAT ___domain (circled with a dashed line), indicating that the putatively assigned SYF3 β-hairpin might alternate between a C19L1-bound and C19L1-unbound conformation. n. and o. AlphaFold2 Multimer PAE and pLDDT plots for the predictions shown in k and l.
Extended Data Fig. 6 Cryo-EM analysis of human ILS spliceosomes.
a. Schematic of purification of TFIP11-bound spliceosomes from human cells. GFP-TFIP11 was overexpressed in K562 suspension cells and TFIP11-bound spliceosomes were purified from 30 L of suspension cell culture. After immunoprecipitation (IP) and elution with 3C protease, spliceosomes were further purified via a sucrose gradient. b. Coomassie-stained SDS-Poly-Acrylamide Gel (SDS-PAGE) of the TFIP11-GFP IP. Bands in the gel are labelled according to the molecular weight of the ILS subunits. This experiment was performed four times. c. Denoised micrograph of gradient-purified and crosslinked Hs ILS, imaged on a Titan Krios G4 with a Falcon 4i detector. d. 2D class averages from the dataset. e. Composite cryo-EM density, obtained from 14 local refinements and filtered by local resolution. Transparent density in the background shows a local refinement map (focused on PAXBP1) low-pass filtered with a gaussian filter with a sigma of three standard deviations. f. Model of the human ILS″, with disassembly factors shown as ribbons and spliceosome core proteins shown in addition as a transparent surface. A difference density, calculated by subtracting simulated model density (low-pass filtered to 20 Å) resolution) from experimental density (ILS consensus refinement map low-pass filtered with a gaussian filter with a sigma of three standard deviations) reveals additional density at the Ce ILS″ C19L1 CWFJ position.
Extended Data Fig. 7 Release of mRNP and spliceosome proteins from the post-catalytic spliceosome unmasks binding sites for the disassembly factors.
a. Overview cartoon, placing the depicted structures into context of the spliceosome disassembly pathway. b. Structural comparison of the structures of the P complex (Model of a Ce P complex based on the updated human P complex, this work), the intron lariat spliceosome immediately after mRNP release (modelled) and the ILS′ (Ce structure, this work). Proteins thar are exchanged in the transition are labelled. Numbers indicate regions for zoom ins in panels c-e. c. Overlay and close-up view of the P-complex structure with the ILS′ reveals a clash of TFIP11 with the EJC (EIF4A3 subunit) and with CWC22, NOSIP, and SRRM2 in the P complex. This clash would occur both in the ILS′ and ILS″. Clashing proteins are outlined in black. d. Overlay and close-up view of the P complex structure with the ILS′ reveals a clash between PPWD1 and PAXBP1 on BRR2. e. Overlay and close-up view of the P complex structure with the ILS″ reveals a clash between C19L2 and the path of the ligated exons in the P-complex.
Extended Data Fig. 8 The ILS″ is competent for disassembly upon ATP addition.
a. Comparison between the structures of DHX15 bound to the G-patch domains of TFIP11 (this study), NKRF1 (ref. 30) (PDB 6SH7), and SUGP1 (ref. 41) (PDB 8EJM). All G-patch domains show an identical binding mode, but additional residues are observed in the TFIP11 G-patch in the Ce ILS″. b. Sequence alignments of the G-patch domains shown in a. c. Schematic of a fluorescence-based helicase assay as described in ref. 72 in which an RNA substrate with 3′ overhang and a 5′ fluorophore label (AlexaFluor588) is annealed to a complementary RNA that carries a fluorescence quencher (black hole quencher, BHQ) at its 3′ end. When the RNA strands are annealed, fluorescence is quenched. Upon separation of the RNA duplex by a helicase, fluorescent signal is increased. To prevent re-annealing, an excess of an unlabeled DNA strand complementary to the AF588-labeled RNA is added (not shown in schematic). Sequences for RNA and DNA used are as in ref. 72. d. The Ce TFIP11 G-patch stimulates DHX15 helicase activity. Helicase assay was performed as shown in panel c. N = 4 replicates were measured. Fluorescence intensities were measured in a plate reader, background corrected, and normalized to the highest value. Error bars show the standard deviation of the mean. P-values from pairwise two-sided t-tests are indicated. e. Denaturing PAGE analysis of the RNAs from the helicase assay shown in panel d after incubation of the RNAs with proteins and ATP. No degradation of the RNA was observed. This experiment was performed three times. f. DHX15 is not bound to ATP in the Ce ILS″ cryo-EM structure. Comparison of DHX15-RNA structure in the ILS″ (left) and a crystal structure of the highly conserved Chaetomium thermophilium (Ct) PRP43 (PDB ID 5LTA, refs. 31,60 % sequence identity between Ce DHX15 and Ct Prp43) bound to RNA and the ATP mimic ADP-BeF3 (middle). In presence of ADP-BeF3, DHX15 adopts a closed conformation, compressing the RNA so that nucleotide +5 (n + 5) is flipped outwards and no longer forms a stacking interaction with the neighboring bases. Right: Overlays of the Ce ILS″ RNA density (transparent red) with the modelled U6 snRNA 3′ end, or the poly-U RNA conformation of Prp43 in presence of ADP-BeF3 indicates that in the Ce ILS″ the RNA is relaxed and DHX15 is ATP-unbound. This is further confirmed by the lack of density in the DHX15 ATP binding pocket in the Ce ILS″ (not shown). g. In vitro ILS disassembly assay. Left: schematic of the assay. Spliceosomes where immobilized on beads via the PRP19-3xFLAG tag, washed, and incubated with ATP. Upon ILS disassembly, components of the Nineteen core complex (NTC core), the Nineteen related complex (NTR) and the U5 snRNP should remain immobilized, while the disassembly factors and U2 snRNP proteins should be depleted from the beads. The bead bound fraction was then analyzed by mass spectrometry. Right: Volcano plot showing differential abundance of proteins with or without ATP treatment. Consistent with the ILS″ structure, the disassembly factors TFIP11, PAXBP1, DHX15, C19L1, C19L2, the NTR subunit SDE2, and the U2 snRNP subunits U2A′ (RU2A) and U2B″ (RU2B) are depleted upon ATP treatment, suggesting that the Ce ILS″, which constitutes a minor fraction of spliceosomes in Ce extract according to cryo-EM particle classification (Supplementary Fig. 1), is competent for in vitro disassembly. ILS subunits are indicated by large circles and color-coded according to subcomplex. The horizontal line at p = 0.05 indicates the commonly used statistical significance cutoff. h. Fold reduction of ILS subunit abundance after incubation with ATP and PRP19-3xFLAG IP as determined by mass spectrometry in panel g.
Extended Data Fig. 9 Genetics in C. elegans support roles of SYF2.
a. Ce SYF2 and Ce SDE2 bind the U2-U6 helix II in the ILS″. U2 snRNA, U6 snRNA, SYF2 and SDE2 are shown as ribbons and DHX15 is shown as an outline. b. An AlphaFold2 Multimer prediction of human SDE2 with SYF2 and SYF1 suggests an identical binding mode of Hs SDE2, however it was not observed in the experimental density due to limited local resolution. c. Sequence alignments of Ce and Hs SDE2 and SYF2. d. Schematic of syf-2 mutant alleles generated by CRISPR-Cas9 in C. elegans. e. Viability of syf-2 mutant animals. Single worms of the indicated genotypes were placed on individual plates at the L3/L4 stage and grown at 20 °C for 96 h. Animals with deletion of helix 1 (Δanchor) are viable as homozygotes, but animals with deletion of helices 1 and 2 (Δanchor+wedge) are only viable as heterozygotes; homozygous mutants are thus progeny of heterozygous mothers. Sterility was scored as the inability to produce numerous progeny that developed into L4 larvae. A few sterile animals still produced <10 embryos or early larvae but these did not develop further. f. Viability of wild-type or syf-2 Δanchor mutant strains treated with empty vector (e.v.) or anti-syf-2 RNAi, at standard (20 °C) or low (15 °C) culture temperatures. Worms were synchronized as L1 larvae, placed on RNAi plates and grown at the corresponding temperatures. Viability was assessed as the total number of F1 progeny that reached the L4 stage. N = 3 animals were analyzed for assays at 15 °C and N = 5 animals were analyzed for assyays at 20 °C. P-values from pairwise two-sided t-tests are indicated. g. Measurement of the synthetic effect of RNAi against sde-2 on syf-2 Δanchor mutant viability. Viability was measured as described in e at both 15 °C and 20 °C. RNAi against mog-7/PAXBP1 was used as a positive control as an essential splicing protein. N = 3 animals were analyzed for assays at 15 °C and N = 5 animals were analyzed for assyays at 20 °C. P-values from pairwise two-sided t-tests are indicated.
Supplementary information
Supplementary Information
This file contains Supplementary Text 1, Supplementary Figs. 1–6, Supplementary Tables 3 (C. elegans strains generated for this study), 4 (DNA sequences used for genome engineering and genotyping of the prp-19 locus) and 5 (DNA sequences used for genome engineering and genotyping of syf-2 alleles), and legends for Supplementary Videos 1–5.
Supplementary Table 1
Guide to coordinate models and cryo-EM maps.
Supplementary Table 2
Overview of C. elegans spliceosome subunit orthologues and their mutant phenotypes.
Supplementary Video 1
Structure of the C. elegans ILS.
Supplementary Video 2
Structure of the C. elegans ILS.
Supplementary Video 3
Integrative model of the human ILS.
Supplementary Video 4
Revised model of the human P complex.
Supplementary Video 5
Model of terminal spliceosome disassembly.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vorländer, M.K., Rothe, P., Kleifeld, J. et al. Mechanism for the initiation of spliceosome disassembly. Nature 632, 443–450 (2024). https://doi.org/10.1038/s41586-024-07741-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07741-1
This article is cited by
-
The green side of splicing: algal spliceosome shows remarkable structural conservation
The EMBO Journal (2025)
-
Structural insights into spliceosome fidelity: DHX35–GPATCH1- mediated rejection of aberrant splicing substrates
Cell Research (2025)
-
Spliceosome-associated quality control
Cell Research (2025)
-
How does the spliceosome dismantle itself?
Nature (2024)