Abstract
Motivations bias our responses to stimuli, producing behavioural outcomes that match our needs and goals. Here we describe a mechanism behind this phenomenon: adjusting the time over which stimulus-derived information is permitted to accumulate towards a decision. As a Drosophila copulation progresses, the male becomes less likely to continue mating through challenges1,2,3. We show that a set of copulation decision neurons (CDNs) flexibly integrates information about competing drives to mediate this decision. Early in mating, dopamine signalling restricts CDN integration time by potentiating Ca2+/calmodulin-dependent protein kinase II (CaMKII) activation in response to stimulatory inputs, imposing a high threshold for changing behaviours. Later into mating, the timescale over which the CDNs integrate termination-promoting information expands, increasing the likelihood of switching behaviours. We suggest scalable windows of temporal integration at dedicated circuit nodes as a key but underappreciated variable in state-based decision-making.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper and can also be found at https://figshare.com/projects/Gautham_Miner_Franco_Thornquist_Crickmore_2024/200581 (ref. 49).
References
Crickmore, M. A. & Vosshall, L. B. Opposing dopaminergic and GABAergic neurons control the duration and persistence of copulation in Drosophila. Cell 155, 881 (2013).
Thornquist, S. C., Langer, K., Zhang, S. X., Rogulja, D. & Crickmore, M. A. CaMKII measures the passage of time to coordinate behavior and motivational state. Neuron 105, 334–345.e9 (2020).
Thornquist, S. C., Pitsch, M. J., Auth, C. S. & Crickmore, M. A. Biochemical evidence accumulates across neurons to drive a network-level eruption. Mol. Cell 81, 675–690.e8 (2021).
Flavell, S. W., Gogolla, N., Lovett-Barron, M. & Zelikowsky, M. The emergence and influence of internal states. Neuron 110, 2545–2570 (2022).
Hindmarsh Sten, T., Li, R., Otopalik, A. & Ruta, V. Sexual arousal gates visual processing during Drosophila courtship. Nature 595, 549–553 (2021).
Ko, K. I. et al. Starvation promotes concerted modulation of appetitive olfactory behavior via parallel neuromodulatory circuits. eLife 4, e08298 (2015).
Aton, S. J. Set and setting: how behavioral state regulates sensory function and plasticity. Neurobiol. Learn. Mem. 106, 1–10 (2013).
Lange, R. D. & Haefner, R. M. Characterizing and interpreting the influence of internal variables on sensory activity. Curr. Opin. Neurobiol. 46, 84–89 (2017).
McLachlan, I. G. et al. Diverse states and stimuli tune olfactory receptor expression levels to modulate food-seeking behavior. eLife 11, e79557 (2022).
Vogt, K. et al. Internal state configures olfactory behavior and early sensory processing in Drosophila larvae. Sci. Adv. 7, eabd6900 (2021).
Richman, E. B., Ticea, N., Allen, W. E., Deisseroth, K. & Luo, L. Neural landscape diffusion resolves conflicts between needs across time. Nature 623, 571–579 (2023).
Wu, Z. et al. Context-dependent decision making in a premotor circuit. Neuron 106, 316–328.e6 (2020).
Burgess, C. R. et al. Hunger-dependent enhancement of food cue responses in mouse postrhinal cortex and lateral amygdala. Neuron 91, 1154–1169 (2016).
Allen, W. E. et al. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364, 253 (2019).
Yasuda, R., Hayashi, Y. & Hell, J. W. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Nat. Rev. Neurosci. 23, 666–682 (2022).
Silva, A. J., Paylor, R., Wehner, J. M. & Tonegawa, S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science 257, 206–211 (1992).
Sebastian Seung, H., Lee, D. D., Reis, B. Y. & Tank, D. W. Stability of the memory of eye position in a recurrent network of conductance-based model neurons. Neuron 26, 259–271 (2000).
Major, G. & Tank, D. Persistent neural activity: prevalence and mechanisms. Curr. Opin. Neurobiol. 14, 675–684 (2004).
Mohammad, F. et al. Optogenetic inhibition of behavior with anion channelrhodopsins. Nat. Methods 14, 271–274 (2017).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Inagaki, H. K. et al. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11, 325–332 (2014).
Seeds, A. M. et al. A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. eLife 3, e02951 (2014).
Namiki, S., Dickinson, M. H., Wong, A. M., Korff, W. & Card, G. M. The functional organization of descending sensory-motor pathways in Drosophila. eLife 7, e34272 (2018).
Tombes, R. M., Faison, M. O. & Turbeville, J. M. Organization and evolution of multifunctional Ca2+/CaM-dependent protein kinase genes. Gene 322, 17–31 (2003).
Park, D., Coleman, M. J., Hodge, J. J. L., Budnik, V. & Griffith, L. C. Regulation of neuronal excitability in Drosophila by constitutively active CaMKII. J. Neurobiol. 52, 24–42 (2002).
Hanson, P. I., Meyer, T., Stryer, L. & Schulman, H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron 12, 943–956 (1994).
Elgersma, Y. et al. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron 36, 493–505 (2002).
Miner, L. E., Gautham, A. K. & Crickmore, M. A. Local desensitization to dopamine devalues recurring behavior. Preprint at bioRxiv https://doi.org/10.1101/2024.02.20.581276 (2024).
Hamada, F. N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008).
Zhuo, Y. et al. Improved green and red GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 21, 680–691 (2024).
Scholz, N. et al. Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons. eLife 6, e28360 (2017).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Huk, A. C. & Shadlen, M. N. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J. Neurosci. 25, 10420–10436 (2005).
Wang, S., Faeder, J. R., Setlow, P. & Li, Y. Q. Memory of germinant stimuli in bacterial spores. mBio 6, e01859–15 (2015).
Wang, K. et al. Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature 589, 577–581 (2021).
Kimura, K., Hachiya, T., Koganezawa, M., Tazawa, T. & Yamamoto, D. Fruitless and Doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759–769 (2008).
Hoopfer, E. D., Jung, Y., Inagaki, H. K., Rubin, G. M. & Anderson, D. J. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 4, e11346 (2015).
Feng, K., Palfreyman, M. T., Häsemeyer, M., Talsma, A. & Dickson, B. J. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron 83, 135–148 (2014).
Zhou, C., Pan, Y., Robinett, C. C., Meissner, G. W. & Baker, B. S. Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163 (2014).
Zhang, S. X., Miner, L. E., Boutros, C. L., Rogulja, D. & Crickmore, M. A. Motivation, perception, and chance converge to make a binary decision. Neuron 99, 376–388.e6 (2018).
Kallman, B. R., Kim, H. & Scott, K. Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 4, e11188 (2015).
Ebitz, R. B. & Hayden, B. Y. The population doctrine in cognitive neuroscience. Neuron 109, 3055–3068 (2021).
Jackman, S. L. & Regehr, W. G. The mechanisms and functions of synaptic facilitation. Neuron 94, 447–464 (2017).
Südhof, T. C. Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol. 4, a011353 (2012).
Zucker, R. S. & Regehr, W. G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Zhang, S. X., Rogulja, D. & Crickmore, M. A. Dopaminergic circuitry underlying mating drive. Neuron 91, 168–181 (2016).
Mussells Pires, P., Zhang, L., Parache, V., Abbott, L. F. & Maimon, G. Converting an allocentric goal into an egocentric steering signal. Nature 626, 808–818 (2024).
Nern, A., Pfeiffer, B. D. & Rubin, G. M. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc. Natl Acad. Sci. U.S.A. 112, E2967–E2976 (2015).
Gautham, A. K., Miner, L. E., Franco, M. N., Thornquist, S. C. & Crickmore, M. A. Data for ‘Dopamine biases decisions by limiting temporal integration’. Figshare https://figshare.com/projects/Gautham_Miner_Franco_Thornquist_Crickmore_2024/200581 (2024).
Acknowledgements
The authors thank D. Rogulja for discussions, comments on the manuscript, and hosting us in her laboratory for the early stages of this project; R. Yasuda and L. Yan for assistance building the 2-photon microscope used in these experiments; J. Zhang, L. Kerrick, R. Persaud, P. Rifkin, A. Singh, M. Hoffman, E. Zheng, M. Dello Russo and G. Verderame for assistance performing experiments related to or inspiring those in this work; B. Pfeiffer, D. Anderson and G. Rubin for sharing the UAS and LexAop2 CsChrimson-tdTomato stocks before publication; O. Mazor and P. Gorelik for technical advice on designing the experimental apparatus; J. Weisman for assistance machining the ex vivo physiology apparatus in Fig. 4b and Extended Data Fig. 7i–l and E. Hacisuleyman for assistance stabilizing the preparation; G. Maimon for hosting S.C.T. during the revision of this work; Y. Li for sharing the 10x-UAS-GRAB-DA3m plasmid ahead of publication; members of the Maimon laboratory for feedback on concepts and experiments; and current and former members of the Crickmore and Rogulja laboratories for comments on the manuscript. This work was funded by the US National Institutes of Health (R01NS111441 and R01GM134222). S.C.T. was supported by a National Science Foundation Graduate Research Fellowship (DGE1144152) and Helen Hay Whitney Postdoctoral Fellowship. L.E.M. was supported by a National Science Foundation Graduate Research Fellowship (DGE2140743).
Author information
Authors and Affiliations
Contributions
A.K.G. and S.C.T. performed the behavioural experiments, except Fig. 3a which was performed by L.E.M. Fly genetics were performed by A.K.G., S.C.T. and M.A.C. The wind presentation chambers were designed and built by A.K.G. A.K.G. performed the imaging experiments, except those in Fig. 4b and Extended Data Fig. 7i–l which were performed and analysed by S.C.T. MANC electron microscopy analysis was performed by S.C.T. Statistical analysis code was written by S.C.T. Linear systems analyses and modelling were performed by S.C.T. M.N.F. wrote the FLIM analysis code based on advice from S.C.T. A.K.G., S.C.T. and M.A.C. wrote the paper, with input from L.E.M. and M.N.F.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jesse Goldberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Sustained CDN activity is necessary and sufficient to end matings.
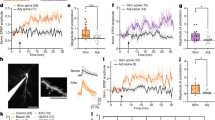
(a) Left: Individual CDNs labelled via MultiColor FlpOut48 (image of a single optical section of the abdominal ganglion). Right: CDN dendrites selectively cover the midline tracts of the abdominal ganglion (blue); the CDNs send axonal projections throughout the abdominal ganglion (magenta). Scale bars are 20 μm. (b) Electrical activity of the CDNs is only necessary around the time of termination to end the mating: silencing that begins just before the natural time of termination (20 min) is still sufficient to prolong the mating –5 flies stopped mating before the light was turned on. (c) Optogenetic stimulation of the CDNs using CsChrimson preceding the onset of mating does not affect copulation duration if only supplied during courtship (brown), but shortens copulation by several minutes if continued into the mating (flashing red light throughout the duration of the experiment, “tonic”, red). These results closely resemble the results of thermogenetic activation in previous work1 that did find immediate termination of the mating upon CDN activation. Providing the same optogenetic activation only after mating begins results in near-immediate termination of copulation (blue). (d) Flies end matings in response to 2 seconds of optogenetic CDN stimulation with varying latencies. Left: ethogram, right: cumulative distribution plot. (e) Stimulation of the CDNs followed by immediate electrical silencing largely prevents the termination of matings that had not ended before the silencing began. Left: ethogram, right: cumulative distribution plot. (f) Mating with a heat-insensitive, Gr28b.d;TrpA1 double mutant female does not change the male’s decision to stop mating when threatened by heat.
Extended Data Fig. 2 Further characterization of integrative properties of multimodal threats during mating.
(a) Flies were placed in elevated behavioral arenas connected to two-way solenoids. Compressed air sources were fed into airflow meters and then into the solenoids, which gate the delivery of the wind. By controlling solenoid opening via Arduino, specific gusts of wind of timed duration can be delivered to behavioral arenas. A camera was suspended above the behavioral arenas. For more information, see Methods. (b) Side view of each well. Tubes were connected into wells via adapters, and a mesh layer was placed over the hole in the floor so the flies did not fall in. (c) Photo of the setup. Solenoids are controlled via a computer connected to the Arduino (not in view). (d) Flies end matings in response to a 350-millisecond wind gust with varying latencies. Cumulative distribution plot of the 10- and 15-minute data in Fig. 2d. (e) Single 650-millisecond wind gusts at 10 min into mating, and 250-millisecond wind gusts at 15 min into mating each terminate ~30% of matings. Data used to calculate independent probability of paired pulse experiment in Fig. 2f. (f) Of 164 descending-interneuron-labeling lines screened, none terminated mating at 5 min when stimulated for 15 seconds with CsChrimson. SS01593 (containing the serotonergic descending neuron DNg26, as well as labeling other cells) is the most effective line at terminating mating at 15 min. (g) DNg26 (SS01593) sends projections to the abdominal ganglion. (h) 700 milliseconds of DNg26 stimulation at 10 min into mating, or 300 milliseconds of DNg26 stimulation at 15 min into mating terminate ~30% of matings. (i) Paired pulse stimulation (700 ms at 10 min, 300 ms at 15 min) of DNg26 is integrated over a longer timescale when delivered at 15 min into mating.
Extended Data Fig. 3 Quantitative estimation of the changing time constant of integration.
(a) If the instantaneous probability of terminating a mating in response to sustained stimulation ramps up as an exponential (top), then the cumulative probability of a mating ending by a particular time into a sustained stimulation follows the function \(\sigma (t)=1-\exp (\,-{p}_{0}\tau (t-(\tau (1-\exp (\,-t/\tau )))))\) (bottom). (b) Parameter estimates for \(\tau \) (time constant, bottom) and \({p}_{0}\) (intensity, top) across timepoints and conditions. \({p}_{0}\) is sensitive to stimulation intensity but not time into mating, while \(\tau \) scales with time into mating, but not stimulation intensity. Error bars show the square root of the estimated parameter variance using the Cramér-Rao bound. (c) Temporal integration is necessary to explain the behavior of flies during sustained optogenetic stimulation, as a model predicting no temporal integration (no \(\tau \)) ascribes a much lower likelihood to the data sets observed (bottom). Temporal integration is also needed to explain the increasing probability of termination as the stimulus goes on (top, data fit with a kernel density estimate). (d) Termination times of CDN>Chr flies exposed to green light for sixty seconds (intensity indicated above graphs). Fitting the cumulative distribution to the model in (a) reveals a close fit. Solid black line: maximum likelihood fit. Error bars: pointwise 95% coverage intervals sampled according to estimated covariance of the parameters. The data used to fit the model for medium intensity light are the same as is plotted in Fig. 2k.
Extended Data Fig. 4 Analysis of the statistical methodologies for measuring temporal integration.
(a) Sampling scheme for generating data sets. \(n\) samples were generated according to the given cumulative distribution function, \({\sigma }_{{p}_{0},\tau }\), and these were used to fit estimates for the generating \(\tau \) and \({p}_{0}\). The value of \(n\) was varied logarithmically from 10 to 1000 to evaluate what sample size would be necessary to accurately estimate the parameters of the distribution. (b) The cumulative distribution function can be qualitatively reconstructed with samples of size ~ 100 across a wide range of cumulative distribution function shapes. Smaller sample sizes (e.g. ~30) are highly variable, especially when the overall number of flies terminating the mating during the stimulation is low (top row). (c) The sensitivity of the inference of the value of \(\tau \) to sample size across a range of \({p}_{0}\) values. The closer a point is to the diagonal, the more likely the fitting procedure is to capture the correct \(\tau \). The fitting procedure overestimates \(\tau \) at low sample sizes, especially when the true value for \(\tau \) is small. This may, to some extent, be explained by the fact that termination times are rounded to the nearest second (we find it is impossible to judge the time of termination more precisely than this value, given the complex motor sequence of terminating the mating). For larger sample sizes, the estimate is much better, so long as a large number of flies terminate the mating during the stimulation. When \({p}_{0}\) and \(\tau \) are both small, however, the inference is considerably less reliable, because these conditions correspond to cases in which very few flies terminate the mating during the stimulus, providing very little information about \(\tau \). (d) As in ©, but instead examining the sensitivity of the estimate of \({p}_{0}\). The parameter \({p}_{0}\) is easier to estimate, because even flies that do not terminate the mating during the stimulation are still informative about its value to some extent (see Methods). However, we find that \({p}_{0}\) is systematically underestimated due to the bias towards overestimating \(\tau \) and the fact that the two estimates show substantial anticovariance (elaborated in panel (e)). (e) Covariance of \({p}_{0}\) and \(\tau \) for various sample sizes. Dashed lines indicate the true parameter values, while independent points show individual sample estimates. The two parameters always anticovary, as indicated by the diagonal slant of each distribution. This reflects the fact that \({p}_{0}\) only appears in the cumulative distribution with \(\tau \) in the form \({p}_{0}\tau \), and so this term is easier to fit than either value alone. If \(\tau \) is overestimated, \({p}_{0}\) will tend to be underestimated to compensate. The multiplicative relationship is clear from the approximately linear covariance of the logarithm of the two parameters. When the data is more informative about \(\tau \), i.e. many flies terminate the mating during the experiment, the cluster is much smaller (e.g. the orange data set). We therefore restricted our experiments to those conditions that would generate reliable estimates of the parameters, especially in cases where we expected \(\tau \) or \({p}_{0}\) to be very small.
Extended Data Fig. 5 Physiological and behavioral consequences of targeted manipulation of CaMKII activity the CDNs.
(a) Constitutively active CaMKII (T287D) has little-to-no effect on the ability of CsChrimson stimulation to evoke calcium transients in the CDNs, as measured by changes in fluorescence of GCaMP6s. Left, middle left: average traces after 2 and 5 seconds of CsChrimson stimulation. Middle right: max fluorescence after stimulation. Right: average residual calcium 15-20 seconds after stimulation. (b) CaMKII T287D in the CDNs cannot suppress the heat threat response with a non-functional catalytic ___domain (K43M). Both the T287D and K43M mutations are contained on the same UAS-CaMKII transgene. (c) CaMKII T287D in the CDNs has no effect on fertility. (d) CaMKII T287D in the CDNs extends mating duration. (e) Overexpression of wildtype CaMKII in the CDNs does not decrease the likelihood of terminating mating in response to a heat threat. (f) Expression of CaMKII T287D selectively in CDN-Gal4 cells that do not also express Tsh-Gal80 prevents the motivational consequences of expression of CaMKII T287D in all CDN-Gal4 cells. (g) Expressing constitutively active CaMKII in the CDNs prevents termination in response to wind. (h) CaMKII knockdown in the CDNs does not alter mating duration. (i) Mating termination is still dependent on CDN electrical activity when CaMKII is knocked down in the CDNs. (j) Left: termination response to 500 ms red light stimulation of the CDNs with CaMKII knockdown. Right: paired pulse response with CaMKII knockdown in the CDNs. (k) Left: termination response to 500 ms red light stimulation of the CDNs with expression of CaMKII T287D. Right: paired pulse response with expression of CaMKII T287D in the CDNs. (l) A single 250 millisecond pulse of CDN stimulation is very unlikely to cause termination regardless of time into mating or CaMKII manipulation. Plotted is the fraction of flies (for cycle lengths: 2.5, 5, and 10 sec) from Fig. 3g that terminated in response to the first of 10 pulses.
Extended Data Fig. 6 Dopamine potentiates CaMKII activity in the CDNs without increasing calcium influx.
(a) CaMKII activity in the axons of the CDNs (as reported by the fluorescence lifetime of the FRET sensor green-Camuiα) decays over ~1 min after 10 seconds of blue light stimulation of the Channelrhodpsin-2 variant ChR2-XXM whereas calcium levels (as measured by changes in the fluorescence of GCaMP6s) decline over ~5 seconds. 3112Laser power was kept at ~5 milliwatts, to limit the basal excitation of ChR2-XXM. Error bar shading for all imaging data represents SEM. (b) CaMKII activity, as reported by change in fluorescence lifetime of green-Camuiα (left), is more strongly activated by transient blue light stimulation of ChR2-XXM in the presence of dopamine perfusion (right). Laser power was kept at ~5 mW, to limit the basal excitation of ChR2-XXM. (c) Left: Dopamine does not potentiate CaMKII activity in another set of Dsx+ neurons (female pC1 neurons25). Right: ~10 mW laser stimulation of ChR2-XXM in pC1 neurons induces an increase in GCaMP6s fluorescence that relaxes to baseline after a few minutes. (d) Constant stimulation is required to keep CaMKII activity high in the presence of dopamine. After stopping laser stimulation for ~100 seconds (“laser break”), CaMKII levels return to baseline and then ramp up again once the laser is turned back on. (e-f) Dopamine does not increase calcium influx in the CDNs. (e) Peak calcium levels (from Fig. 4e) in response to ~10 mW laser stimulation of ChR2-XXM before and after dopamine perfusion. (f) Left: average traces of 2.5 seconds blue light stimulation of ChR2-XXM with saline and dopamine perfusion. Middle: peak calcium after stimulation. Right: residual calcium 15–20 seconds after stimulation (note the small y-axis to highlight a potential effect on residual calcium). (g) Dopamine potentiates CaMKII activation under continuous ~10 mw infrared laser ChR2-XXM stimulation.
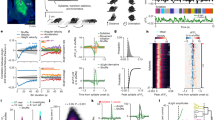
Extended Data Fig. 7 Characterization of the influence of abdominal ganglion circuitry (Crz neurons and dopaminergic neurons) on the CDNs.
a) The Crz neurons project throughout the abdominal ganglion, with processes closely apposed to those of the CDNs, both near their axons (left) and dendrites (right), though synaptic connectivity cannot be concluded. (b) Optogenetic stimulation of the CDNs while the Crz neurons are silenced results in termination of the mating, demonstrating that the CDNs operate downstream of the Crz neurons in determining the motivational state of the fly. (c) Silencing the Crz neurons reduces the response to sustained stimulation of the CDNs by selectively decreasing the gain on the input (~8-fold), leaving the time constant of integration largely unaffected. (d) Cumulative distribution functions used for estimating the parameters of panel (c). (e) For the first ~6 min of mating, high CaMKII activity in the Corazonin neurons prevents the network eruption that triggers sperm transfer2,3. Before the eruption, males that have not mated recently are impervious to challenges of apparently all varieties and severities. At 6 min, the eruption increases \({p}_{0}\), allowing threat information to be delivered to the Copulation Decision Neurons (CDNs), through mechanisms not yet understood. After the eruption, dopaminergic inputs to the CDNs increase intracellular CaMKII levels to restrict \(\tau \), the timescale of competing information retention. (f) Dopaminergic neurons (orange) send projections throughout the abdominal ganglion, often forming varicosities near CDN (green) processes (indicated by white arrowheads). Images are obtained from a single optical plane. (g) Silencing the dopaminergic neurons does not affect overall copulation duration. (h) Warmth alone decreases τ but increases p0 showing that heat cannot account for the effects of stimulation the dopaminergic neurons. (i) Data as in Fig. 4b but plotting lifetime instead of fluorescence. The measurement was more variable but there is a consistent ~200 picosecond increase in lifetime with thermogenetic stimulation. (j) Thermogenetic stimulation of the dopaminergic neurons of the abdominal ganglion results in an increase in fluorescence and fluorescence lifetime. Note that 1) the lifetime is not linearly related to the increase in fluorescence (and so the two measures have differential sensitivity across concentration) and 2) both signals begin to decrease before the temperature is decreased. Because bath application of dopamine resulted in stable fluorescence, this seems unlikely to be bleaching of the indicator. We speculate it results either from habituation of the TrpA1 channel or rapid depletion of the dopaminergic neurons, at least at the scale of the sensor’s dynamic range. Allowing several minutes of recovery at 20 °C permitted a second stimulation to be equally efficacious (not shown). (k) Warming the abdominal ganglion without expression of TrpA1 in dopaminergic neurons resulted in a small but consistent decrease in both fluorescence lifetime and fluorescence itself. The GRAB-DA3m protein itself is likely temperature sensitive, but the effect of temperature produces a change in fluorescence signal opposite to that observed during stimulation of dopaminergic neurons, arguing that signals as in Fig. 4b are not artifacts of the temperature ramp. (l) Bath application of dopamine in the concentration range used in Fig. 4 results in increases in fluorescence lifetime and fluorescence quantitatively similar to that evoked by thermogenetic stimulation of dopaminergic neurons, arguing that these bath concentrations result in physiologically-plausible exposure to dopamine at the CDN axons. These values differ substantially from the reported sensitivity of GRAB-DA3m30 which we speculate arises from the protective glial sheath surrounding the abdominal ganglion, which may buffer the exogenous dopamine levels or rapidly degrade it. Supporting this conclusion, in unpublished experiments, we found that pipette administration of dopamine to a still bath (rather than continuous perfusion) only transiently increased the excitability of CaMKII, unlike the sustained excitability increase observed in Fig. 4.
Extended Data Fig. 8 Identification of putative CDNs.
(a) The 54 predicted-GABAergic neurons of the abdominal ganglion that superficially resemble the CDNs cluster into five groups, as well as a miscellaneous collection, by comparing the proportions and identities of their various synaptic inputs. Left: the cosine similarity of the vector of synaptic inputs for each cell. Right: Each putative CDN’s synaptic inputs across the collection of cells innervating any of the 54 possible candidates. (b) As in a, but for the synaptic output vectors of the CDN candidates (c) IHC of central planes of the abdominal ganglion highlights two features of the CDNs: synaptic release sites along the dorsolateral AG and inputs in the central AG. (d) The overlaid anatomy of the 7 neurons identified in Cluster 2, presumed to be the CDNs, from three perspectives. Left: viewed from the fly’s right side. Middle: viewed from the ventral side of the fly, Right: viewed from the anterior side of the fly. (e) The anatomy of each individual CDN is varied, innervating different portions of the antero-posterior extent of the later abdominal ganglion.
Extended Data Fig. 9 Local interneuron inputs to the putative CDNs are highly varied and likely inhibitory.
(a) The 24 strongest inputs to the CDNs from within the AG show highly varied anatomy, rather than resembling any specific cell class. (b) The neurons with the most ambiguous neurotransmitter predictions (circled in panel a) do not have anatomy closely approximating the labeling by our TH-Gal4 line. (c) The neurons that most strongly innervate the CDNs from within the AG are predicted to be inhibitory (either through GABA or glutamate) and do not receive reciprocal synapses from the CDNs, again arguing against a primary role for recurrence in their ability to integrate inputs. (d) Each individual cell in Clusters 1 and 2 of Extended Data Fig. 8 receives relatively little reciprocal innervation from its postsynaptic targets, especially as compared to the other GABAergic neuron classes. (e) Pooling the CDNs suggests an increase in reciprocal innervation across the cell class, suggesting that many post-synaptic targets of each putative CDN innervates other CDNs. The diagonal of these plots is still much less dense in Clusters 1 and 2, implying that these cells are less recurrently connected than other morphologically-similar interneurons of the AG.
Extended Data Fig. 10 Putative CDNs principally target abdominal ganglion interneurons, and receive few direct recurrent inputs.
(a) The 100 cells receiving the most input from the presumed CDNs make up over 80% of all output synapses and can be divided into three classes: interneuropil neurons of the VNS, local interneurons of the abdominal ganglion, and motor neurons descending the abdominal trunk nerve. (b) The interneuropil targets of the CDNs innervate all three leg neuropil. (c) Most interneuropil targets of the CDNs that receive a substantial amount of CDN input do not innervate the abdominal ganglion, and instead transmit information to all of the leg neuropil (rather than innervating a single neuropil). (d) The abdominal ganglion interneurons targeted by the CDNs densely innervate both halves of the AG with very little input or output in the medial third of the ganglion. (e) The local interneurons of the AG do not strongly reciprocate synaptic input from the CDNs, though a subset of neurons weakly predicted to be GABAergic do synapse back onto the CDNs, providing the opportunity for recurrence through disinhibition. (f) The minority descending neuron output of the CDNs. (g) Motor neurons targeted by the CDNs receive only a very small fraction of their inputs from the CDNs. Most interneuropil neurons receive <10% of their synaptic input from the CDNs. In contrast, many AG interneurons receive a large fraction of their input from the CDNs. (h) Trans-synaptic labeling of CDN targets identifies six large AG interneurons, matching the number of major interneuron targets in the EM volume, but mostly fails to resolve motor or interneuropil neurons.
Extended Data Fig. 11 Putative CDNs receive integrative inputs from the brain and other VNS neuropil.
(a) The 150 primary inputs to the CDNs are divided into four classes: interneuropil neurons of the VNS, local interneurons of the abdominal ganglion, descending neurons from the brain, and ascending sensory neurons from the periphery. (b) Most neurons that target the CDNs target them indiscriminately, with little preferential innervation of individual cells, arguing that the functional unit of the CDNs is the collective, and that their individual differences are not a primary feature. (c,d) Descending neurons from the brain targeting the CDNs typically innervate other neuropil as well. (e) A few descending neurons preferentially target the CDNs, with ¼ to ½ of their synapses being specific to the CDNs, but other direct descending input to the CDNs is minimal. (f,g) A quarter of input synapses to the CDNs come from interneuropil neurons of the VNS that receive input from multiple other leg neuropil (h) Four interneuropil neurons heavily innervate the CDNs, making up their four largest inputs, and two of these almost exclusively synapse on the CDNs (dotted black line is the unity line, indicating all synapses being restricted to the CDNs) (i) The four interneuropil neurons targeting the CDNs target them mostly indiscriminately, rather than singling out individual CDNs (other than hemispheric preferences). (j) The interneuropil neurons innervating the CDNs receive input from multiple cell classes in other neuropil, with some pooling hundreds of synapses from descending neurons coming from the brain.
Extended Data Fig. 12 EM analysis of putative Corazonin neurons.
(a) Four neurons in the MANC volume closely resembling the morphology of the Crz neurons. Individual release sites (localized to both sides of the AG) shown in pink. Three perspectives are shown: from the right side of the fly (left column), from the ventral side of the fly (central column), and from the anterior side (right column). (b) Single cell labeling of individual Crz neurons using a heat-shock induced recombinase. Data from Thornquist et al.3. (c) Cosine similarity of the synaptic output vectors of six Crz-like neurons (body IDs shown) suggests a set of four neurons are approximately equally similar to one another and a separate pair that is more similar internally than to the other two. (d) Most postsynaptic targets of the four Crz-like neurons identified are shared. (e) Most of the Crz neurons’ postsynaptic targets are local neurons of the abdominal ganglion. ~30% of their output synapses are onto descending cells, likely to drive ejaculation and the accompanying abdominal movements. 10% of their synapses are reciprocal (innervating one another), and ~50% of their outputs are onto many different cell classes in the AG. (f) Top: One of the primary classes of neurons targeted by the putative Crz neurons is a set of enervating neurons projecting down the abdominal trunk nerve. These neurons resemble the ejaculation-driving Tph2/CrzR expressing cells from Tayler et al. Bottom: The GABAergic neurons targeted by the Crz neurons are largely restricted to the posterio-lateral portion of the AG, but predominantly do not appear CDN-like. (g) A large fraction of the inputs the Crz neurons receive come from either other Crz neurons, or four other local neurons of the AG. (h) The primary source of synaptic input to the Crz neurons are four peptidergic cells in the AG resembling the neurons labeled by Dh44-Gal4 (unpublished data).
Supplementary information
Supplementary Information
Supplementary Discussion 1, Supplementary Notes 1 and 2, Supplementary Video legends and Supplementary Tables 1 – 4
Supplementary Video 1
Silencing the CDNs prevents the termination of mating in response to heat threats.
Supplementary Video 2
Stimulation of the CDNs terminates the mating with variable latency.
Supplementary Video 3
The termination procedure in response to threats resembles that of stimulation of the CDNs.
Supplementary Video 4
A short gust of wind strongly disturbs mating flies, but does not mechanically dislodge the male from the female.
Supplementary Video 5
Constitutively silencing the CDNs with tetanus toxin prevents termination in response to a gust of wind.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gautham, A.K., Miner, L.E., Franco, M.N. et al. Dopamine biases decisions by limiting temporal integration. Nature 632, 850–857 (2024). https://doi.org/10.1038/s41586-024-07749-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07749-7