Abstract
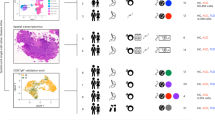
Tissue-selective chemoattractants direct lymphocytes to epithelial surfaces to establish local immune environments, regulate immune responses to food antigens and commensal organisms, and protect from pathogens. Homeostatic chemoattractants for small intestines, colon and skin are known1,2, but chemotropic mechanisms selective for respiratory tract and other non-intestinal mucosal tissues remain poorly understood. Here we leveraged diverse omics datasets to identify GPR25 as a lymphocyte receptor for CXCL17, a chemoattractant cytokine whose expression by epithelial cells of airways, upper gastrointestinal and squamous mucosae unifies the non-intestinal mucosal tissues and distinguishes them from intestinal mucosae. Single-cell transcriptomic analyses show that GPR25 is induced on innate lymphocytes before emigration to the periphery, and is imprinted in secondary lymphoid tissues on activated B and T cells responding to immune challenge. GPR25 characterizes B and T tissue resident memory cells and regulatory T lymphocytes in non-intestinal mucosal tissues and lungs in humans and mediates lymphocyte homing to barrier epithelia of the airways, oral cavity, stomach, and biliary and genitourinary tracts in mouse models. GPR25 is also expressed by T cells in cerebrospinal fluid and CXCL17 by neurons, suggesting a role in central nervous system (CNS) immune regulation. We reveal widespread imprinting of GPR25 on regulatory T cells, suggesting a mechanistic link to population genetics evidence that GPR25 is protective in autoimmunity3,4. Our results define a GPR25–CXCL17 chemoaffinity axis with the potential to integrate immunity and tolerance at non-intestinal mucosae and the CNS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw and processed scRNA-seq data generated in this study are available from the NCBI Gene Expression Omnibus repository under the accession number GSE273397. Supplementary Table 1 lists all published external datasets used in this study. Integrated scRNA-seq datasets used for the analyses can be accessed at http://med.stanford.edu/butcherlab/data/GPR25.html. Source data are provided with this paper.
Code availability
Code for computational analyses is available upon request.
References
Kunkel, E. J. & Butcher, E. C. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1–4 (2002).
Ocon, B. et al. A mucosal and cutaneous chemokine ligand for the lymphocyte chemoattractant receptor GPR15. Front. Immunol. 8, 1111 (2017).
Ricaño-Ponce, I. et al. Refined mapping of autoimmune disease associated genetic variants with gene expression suggests an important role for non-coding RNAs. J. Autoimmun. 68, 62–74 (2016).
Robinson, P. C. et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol. 67, 140–151 (2015).
Habtezion, A., Nguyen, L. P., Hadeiba, H. & Butcher, E. C. Leukocyte trafficking to the small intestine and colon. Gastroenterology 150, 340–354 (2016).
Imaoka, K. et al. Nasal immunization of nonhuman primates with simian immunodeficiency virus p55gag and cholera toxin adjuvant induces Th1/Th2 help for virus-specific immune responses in reproductive tissues. J. Immunol. 161, 5952–5958 (1998).
Sato, A. et al. Vaginal memory T cells induced by intranasal vaccination are critical for protective T cell recruitment and prevention of genital HSV-2 disease. J. Virol. 88, 13699–13708 (2014).
Stary, G. et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348, aaa8205 (2015).
Labuda, J. C. et al. Circulating immunity protects the female reproductive tract from Chlamydia infection. Proc. Natl Acad. Sci. USA 118, e2104407118 (2021).
Tordesillas, L. & Berin, M. C. Mechanisms of oral tolerance. Clin. Rev. Allergy Immunol. 55, 107–117 (2018).
Choi, J. et al. TREGking from gut to brain: the control of regulatory T cells along the gut-brain axis. Front. Immunol. 13, 916066 (2022).
Ellwardt, E., Walsh, J. T., Kipnis, J. & Zipp, F. Understanding the role of T cells in CNS homeostasis. Trends Immunol. 37, 154–165 (2016).
Hu, D. & Weiner, H. L. Unraveling the dual nature of brain CD8+ T cells in Alzheimer’s disease. Mol. Neurodegener. 19, 16 (2024).
Pan, S. et al. Brain Catalog: a comprehensive resource for the genetic landscape of brain-related traits. Nucleic Acids Res. 51, D835–D844 (2023).
Park, J. E. et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 367, eaay3224 (2020).
Billiet, L. et al. Single-cell profiling identifies a novel human polyclonal unconventional T cell lineage. J. Exp. Med. 220, e20220942 (2023).
Dermadi, D. et al. Exploration of cell development pathways through high-dimensional single cell analysis in trajectory space. iScience 23, 100842 (2020).
Gao, X. & Cockburn, I. A. The development and function of CD11c+ atypical B cells – insights from single cell analysis. Front. Immunol. 13, 979060 (2022).
Sigmundsdottir, H. & Butcher, E. C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 9, 981–987 (2008).
Swaminathan, G. et al. The aryl hydrocarbon receptor regulates expression of mucosal trafficking receptor GPR15. Mucosal Immunol. 14, 852–861 (2021).
Xiong, L. et al. Ahr-Foxp3-RORγt axis controls gut homing of CD4+ T cells by regulating GPR15. Sci. Immunol. 5, eaaz7277 (2020).
Mikhak, Z., Strassner, J. P. & Luster, A. D. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J. Exp. Med. 210, 1855–1869 (2013).
Aleotti, A., Goulty, M., Lewis, C., Giorgini, F. & Feuda, R. The origin, evolution, and molecular diversity of the chemokine system. Life Sci. Alliance 7, e202302471 (2024).
Ngo, T. et al. RETRACTED ARTICLE: Orphan receptor ligand discovery by pickpocketing pharmacological neighbors. Nat. Chem. Biol. 13, 235–242 (2017).
Lee, W. Y., Wang, C. J., Lin, T. Y., Hsiao, C. L. & Luo, C. W. CXCL17, an orphan chemokine, acts as a novel angiogenic and anti-inflammatory factor. Am. J. Physiol. Endocrinol. Metab. 304, E32–E40 (2013).
Burkhardt, A. M. et al. CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J. Immunol. 188, 6399–6406 (2012).
Weinstein, E. J. et al. VCC-1, a novel chemokine, promotes tumor growth. Biochem. Biophys. Res. Commun. 350, 74–81 (2006).
Pisabarro, M. T. et al. Cutting edge: novel human dendritic cell- and monocyte-attracting chemokine-like protein identified by fold recognition methods. J. Immunol. 176, 2069–2073 (2006).
Choreño-Parra, J. A., Thirunavukkarasu, S., Zúñiga, J. & Khader, S. A. The protective and pathogenic roles of CXCL17 in human health and disease: potential in respiratory medicine. Cytokine Growth Factor Rev. 53, 53–62 (2020).
Giblin, S. P. & Pease, J. E. What defines a chemokine? – The curious case of CXCL17. Cytokine 168, 156224 (2023).
Oka, T. et al. CXCL17 attenuates imiquimod-induced psoriasis-like skin inflammation by recruiting myeloid-derived suppressor cells and regulatory T cells. J. Immunol. 198, 3897–3908 (2017).
Binti Mohd Amir, N. A. S. et al. Evidence for the existence of a CXCL17 receptor distinct from GPR35. J. Immunol. 201, 714–724 (2018).
Park, S.-J., Lee, S.-J., Nam, S.-Y. & Im, D.-S. GPR35 mediates lodoxamide-induced migration inhibitory response but not CXCL17-induced migration stimulatory response in THP-1 cells; is GPR35 a receptor for CXCL17? Br. J. Pharmacol. 175, 154–161 (2018).
Ding, J. et al. CXCL17 induces activation of human mast cells via MRGPRX2. Allergy https://doi.org/10.1111/all.16036 (2024).
White, C. W. et al. CXCL17 is an allosteric inhibitor of CXCR4 through a mechanism of action involving glycosaminoglycans. Sci. Signal. 17, eabl3758 (2024).
Laudanna, C., Campbell, J. J. & Butcher, E. C. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science 271, 981–983 (1996).
Wyrożemski, Ł. & Qiao, S.-W. Immunobiology and conflicting roles of the human CD161 receptor in T cells. Scand. J. Immunol. 94, e13090 (2021).
Lazarus, N. H. et al. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 170, 3799–3805 (2003).
Srivastava, R. et al. CXCL17 chemokine-dependent mobilization of CXCR8+CD8+ effector memory and tissue-resident memory T cells in the vaginal mucosa is associated with protection against genital herpes. J. Immunol. 200, 2915–2926 (2018).
Hernández-Ruiz, M. et al. Cxcl17−/− mice develop exacerbated disease in a T cell-dependent autoimmune model. J. Leukoc. Biol. 105, 1027–1039 (2019).
Kim, J. et al. Spontaneous proliferation of CD4+ T cells in RAG-deficient hosts promotes antigen-independent but IL-2-dependent strong proliferative response of naïve CD8+ T cells. Front. Immunol. 9, 1907 (2018).
Pruenster, M. et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat. Immunol. 10, 101–108 (2009).
Wein, A. N. et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J. Exp. Med. 216, 2748–2762 (2019).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Ehrhardt, G. R. et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med. 202, 783–791 (2005).
Alon, R. et al. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat. Rev. Immunol. 21, 49–64 (2021).
Liu, J. Z. et al. Dense fine-mapping study identifies new susceptibility loci for primary biliary cirrhosis. Nat. Genet. 44, 1137–1141 (2012).
Odoardi, F. et al. T cells become licensed in the lung to enter the central nervous system. Nature 488, 675–679 (2012).
Skarnes, W. C. et al. A conditional knockout resource for the genome–wide study of mouse gene function. Nature 474, 337–342 (2011).
Kim, E., Tran, M., Sun, Y. & Huh, J. R. Isolation and analyses of lamina propria lymphocytes from mouse intestines. STAR Protoc. 3, 101366 (2022).
Steinert, E. M. et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015).
Cumba Garcia, L. M., Huseby Kelcher, A. M., Malo, C. S. & Johnson, A. J. Superior isolation of antigen-specific brain infiltrating T cells using manual homogenization technique. J. Immunol. Methods 439, 23–28 (2016).
Sumida, H. et al. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci. Immunol. 2, eaao1135 (2017).
Allen, S. J., Hamel, D. J. & Handel, T. M. A rapid and efficient way to obtain modified chemokines for functional and biophysical studies. Cytokine 55, 168–173 (2011).
Lazar, G. A., Desjarlais, J. R. & Handel, T. M. De novo design of the hydrophobic core of ubiquitin. Protein Sci. 6, 1167–1178 (1997).
Zabel, B. A. et al. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 280, 34661–34666 (2005).
Honda, S. et al. Ligand-induced adhesion to activated endothelium and to vascular cell adhesion molecule-1 in lymphocytes transfected with the N-formyl peptide receptor. J. Immunol. 152, 4026–4035 (1994).
Sikkema, L. et al. An integrated cell atlas of the lung in health and disease. Nat. Med. 29, 1563–1577 (2023).
Yoshida, M. et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature 602, 321–327 (2022).
Dominguez Conde, C. et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science 376, eabl5197 (2022).
He, P. et al. A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. Cell 185, 4841–4860.e4825 (2022).
He, S. et al. Single-cell transcriptome profiling of an adult human cell atlas of 15 major organs. Genome Biol. 21, 294 (2020).
Schalck, A. et al. Single-cell sequencing reveals trajectory of tumor-infiltrating lymphocyte states in pancreatic cancer. Cancer Discov. 12, 2330–2349 (2022).
Chang, L. et al. Single-cell clonal tracing of glandular and circulating T cells identifies a population of CD9+ CD8+ T cells in primary Sjogren’s syndrome. J. Leukoc. Biol. https://doi.org/10.1093/jleuko/qiad071 (2023).
Piehl, N. et al. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell 185, 5028–5039.e5013 (2022).
Peng, T. et al. Distinct populations of antigen-specific tissue-resident CD8+ T cells in human cervix mucosa. JCI Insight 6, e149950 (2021).
Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021).
Saluzzo, S. et al. Delayed antiretroviral therapy in HIV-infected individuals leads to irreversible depletion of skin- and mucosa-resident memory T cells. Immunity 54, 2842–2858.e2845 (2021).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7, eabh2169 (2021).
Sjostedt, E. et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367, eaay5947 (2020).
Siletti, K. et al. Transcriptomic diversity of cell types across the adult human brain. Science 382, eadd7046 (2023).
Allen, W. E., Blosser, T. R., Sullivan, Z. A., Dulac, C. & Zhuang, X. Molecular and spatial signatures of mouse brain aging at single-cell resolution. Cell 186, 194–208.e118 (2023).
Matson, K. J. E. et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat. Commun. 13, 5628 (2022).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Hao, Y. et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 42, 293–304 (2024).
Lun, A. T., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res. 5, 2122 (2016).
McCarthy, D. J., Campbell, K. R., Lun, A. T. & Wills, Q. F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186 (2017).
Haghverdi, L., Lun, A. T. L., Morgan, M. D. & Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 36, 421–427 (2018).
Lim, H. S. & Qiu, P. Quantifying cell-type-specific differences of single-cell datasets using uniform manifold approximation and projection for dimension reduction and Shapley Additive exPlanations. J. Comput. Biol. 30, 738–750 (2023).
van Dijk, D. et al. Recovering gene interactions from single-cell data using data diffusion. Cell 174, 716–729.e727 (2018).
King, H. W. et al. Single-cell analysis of human B cell maturation predicts how antibody class switching shapes selection dynamics. Sci. Immunol. 6, eabe6291 (2021).
Monaco, G. et al. RNA-seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. 26, 1627–1640.e1627 (2019).
Ngo, T. et al. Orphan receptor ligand discovery by pickpocketing pharmacological neighbors. Nat. Chem. Biol. 13, 235–242 (2017).
Uhlen, M. et al. The human secretome. Sci. Signal. https://doi.org/10.1126/scisignal.aaz0274 (2019).
Kinsella, R. J. et al. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database (Oxford) 2011, bar030 (2011).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Shiryev, S. A., Papadopoulos, J. S., Schaffer, A. A. & Agarwala, R. Improved BLAST searches using longer words for protein seeding. Bioinformatics 23, 2949–2951 (2007).
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Senior, A. W. et al. Improved protein structure prediction using potentials from deep learning. Nature 577, 706–710 (2020).
Abagyan, R. & Totrov, M. Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and proteins. J. Mol. Biol. 235, 983–1002 (1994).
Acknowledgements
This study was funded by NIH grants no. R01 AI178113 and no. R01 AI047822, MERIT award no. I01 BX-002919 from the United States Department of Veterans Affairs Biomedical Laboratory R&D Service (VA BLR&D), grant no. 1903-03787 from The Leona M. & Harry B. Helmsley Charitable Trust and the Regents of the University of California Tobacco Related Disease Research Program (TRDRP) grants no. T31IP1880 and no. T33IR6609 to E.C.B.; grants no. R21 AI149369 and no. R21 AI156662 to I.K.; grants no. R01 AI161880 and no. R01 GM136202 to I.K. and T.H.; and grant no. R01 MH125244 to S.M. B.A.Z. was supported by Merit Review Award Number I01 BX004115 from the VA BLR&D and by TRDRP grants no. T32IP5349 and no. T33IP6514. A.A. was supported by the California Institute for Regenerative Medicine (CIRM), award no. EDUC2-12677. F.M. was supported by the Department of Excellence 2023–2028 DNBM, the Cariverona Foundation–Research and Development Grant 2022 and the #NEXTGENERATIONEU and the Italian Ministry of University and Research, National Recovery and Resilience Plan (PNRR), project MNESYS (grant no. PE0000006). M.X. was supported by the TRDRP grant no. T31FT1867. Y.B. and B.O. were Research Fellow Awardees of the Crohn’s and Colitis Foundation of America (grants no. 835171 and no. 574148), and B.O. was a postdoctoral fellow of the Ramon Areces Foundation (Madrid, Spain). We thank Y. Yao for statistics, B. Xu for technical advice, G. Ramos for mouse colony maintenance and L. Magalhaes for administrative support. The schematic of GPR25 in Fig. 3a created with Biorender (https://BioRender.com).
Author information
Authors and Affiliations
Contributions
B.O., Y.B., A.A., M.K., M.H., J.P., M.X., K.B., M.L., N.L. and F.M. performed experiments. M.X. and K.B. performed scRNA-seq analysis. M.X., C.Z., J.R.D.D., I.K. and J.P. performed in silico analysis and protein modelling. S.T. performed RNAscope. S.M. and T.S. performed population genetics analyses. J.E.H. collected autopsy samples. M.H. and T.H. provided reagents. E.C.B., M.X., B.O. and Y.B. wrote, and T.H., B.A.Z. and K.B. edited, the manuscript. E.C.B. and J.P. conceived and E.C.B. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Andrew Luster and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Tissue- and subset-selective expression of GPR25 by lymphocytes.
a. Comparison of GPR25, GPR15 and CCR9 in the airway, colon, and SI, respectively, in total CD4 or CD8 T cells. Data from healthy adult and pediatric donors (n = 12–16). Boxplots of GPR25, GPR15 and CCR9 mean imputed expression per patient sample in total CD4 or CD8 T cells are shown, with each dot representing the mean value per sample. Hinges of box correspond to the first and third quartiles. Whisker extends from the corresponding hinge to the max/min value no further than 1.5x interquartile range from the hinge. Samples with fewer than 10 cells are not plotted. *: p-value < 0.05; ****: p-value < 0.0001, two-tailed T-test. b. Normalized transcript per million (TPM) of GPR25 from scRNAseq of all human cell types from the Human Protein Atlas. C. Mean TPM of GPR25 from bulk RNAseq of immune cell types sorted from PBMC of 4 healthy donors. Sample source information is provided in Supplementary Table 1.
Extended Data Fig. 2 GPR25 expression and association with neurodegeneration.
scRNAseq violin plots of imputed GPR25 expression in T cell subsets and myeloid cells in CSF samples from healthy donors and patients with mild cognitive impairment/Alzheimer’s Disease (MCI/AD). Data from all patients with more than 1000 cells are presented with means of individual donors (open circles) and mean values of the donor means (solid circles) ± SEM (n = 28). *: p-value < 0.05; **: p-value < 0.01; ****: p-value < 0.0001; n.s.: non-significant, multivariate regression. Trending differences between healthy and diseased samples are not statistically significant. Sample source information is provided in Supplementary Table 1.
Extended Data Fig. 3 Expression of GPR25 in T cells in the MLN, TLN and small intestines.
a. GPR25+ cells are enriched in mature Tregs during CD4 T cell differentiation in MLN and TLN. CD4 T cells aligned along a developmental path from CD4 naive cells illustrating sequential expression of CCR9 and GPR25 by T cells along a developmental (pseudotime) trajectory seeded from naive CD4 cells. Mature FOXP3-high IL2RA-hi Tregs (Treg hi) emerge late and are enriched in GPR25 + CCR9+ cells in MLN. Cells are pooled from 14 MLN and 9 TLN samples from healthy donors. b. GPR25 is expressed by subsets of Treg and TEM in the small intestines. Violin plots illustrating CCR9 and GPR15 expression by GPR25 + (GPR25 > 0.2) vs GPR25- (GPR25 < 0.2) T cells in SI, pooled from 12 healthy donors and presented with means of individual donors (open circles) and mean values of donor means (solid circles) ± SEM. All gene expression imputed. Sample source information is provided in Supplementary Table 1.
Extended Data Fig. 4 Predicted structure of the GPR25 complex with CXCL17.
a. The overall view of the complex. Receptor and the CXCL17 C-terminal helix are shown in white and black ribbons, respectively, and viewed along the plane of the membrane. b. The acidic C-terminus of CXCL17 is predicted to insert into the predominantly positively charged orthosteric binding pocket of GPR25. The receptor is viewed along the plane of the membrane as in (A) and is shown as a cut-away space-filling mesh colored by electrostatic potential (blue: positive, red: negative). The C-terminal part of CXCL17 is shown as black ribbon (backbone) and sticks (for the carboxyl group and residue side-chains only). c. The amino-acid residue environment in the receptor binding pocket is complementary to the molecular composition of the distal C-terminus of CXCL17, which ensures favorable hydrophobic packing against W952.60 and prominent hydrogen bonding interactions with the network of S1163.29, R1784.64, E19345.52, and R2646.55. Receptor is viewed across the plane of the membrane from the extracellular side and shown in white ribbon and sticks; the two C-terminal residues of CXCL17 are shown in black. Cyan dotted lines denote hydrogen bonds. The model was built using AlphaFold 2.3.2 Multimer89,90,91. Structure was refined and visualized in ICM 3.9-3b92.
Extended Data Fig. 5 CXCL17 is a chemoattractant ligand for GPR25 but not GPR15 or CMKLR1.
a. Human GPR15 transfectants migration to GPR15LG (250 nM) and CXCL17 (10–300 nM). b. Human CMKLR1 transfectants migration to chemerin and CXCL17 (10–300 nM). c. Checkerboard assay with human 4CysCXCL17 250 nM and human GPR25 transfectants. d. Pertussis toxin (100 ng/ml, 2 h pre-treatment before migration assay) inhibits CXCL17-induced chemotaxis on human GPR25 L1-2 transfectants. e. Intact mouse 6CysCXCL17 (3 nM - 1μM) is an active chemoattractant on human GPR25. f. Intact human 4CysCXCL17 is an active chemoattractant on mouse GPR25. g. mGPR25 transduced cells, but not the empty vector transduced counterparts robustly chemotax to mouse and human CXCL17 in in vitro transwell-based migration assays. Results with 3–9 replicates pooled from at least two independent experiments are shown as mean ± SEM. ****; P < 0.0001 vs no chemokine control in a two-tailed T-test.
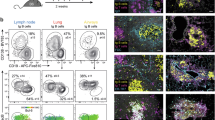
Extended Data Fig. 6 Subset selective T cell chemotaxis to CXCL17.
a. Table showing % of migration to no chemokine in Fig. 4a and c. b-d. Tonsil cells were migrated in transwells to human 4CysCXCL17 or human GPR15LG for 3 hrs. Migrated and input cells were counted and phenotyped by flow cytometry. b-d. Naive (CD45RO− CD45RA +) or indicated effector/memory (CD45RO + CD45RA−) TCRαβ + CD4+ subsets were defined with MAbs to intracellular Foxp3 and CD25 (Tregs), and CD161, a marker of mucosal tissue homing T cells. Mucosal-associated invariant T cells are Vα7.2 +. NK cells were defined as CD14−, HLA/DR−, CD3−, CD19−, CD56 +, CD16−. NKT shared the same immunophenotyping but were gated as CD3 +. Conventional dendritic cells (DC) were defined as CD3−, CD19−, CD14−, HLA/DR + , CD11c +. Plasmacytoid dendritic cells (pDC) were defined as CD3−, CD19−, CD14−, HLA/DR +, CD123 +. Data are % of input cells migrated above mean “no chemokine/NC control” migration (which defines 0). e. Table showing % of migration to no chemokine in panels b-d. Results pooled from three independent experiments and shown as mean ± SEM of % of specific migration, except for hGPR15LG (two experiments). N ≥ 5. *; P < 0.05, **; p < 0.01, ***; p < 0.001, ****; p < 0.0001. One way ANOVA analysis with Dunnet post hoc test was performed to each cell subset comparing the indicated condition vs no chemokine control (NC).
Extended Data Fig. 7 CXCL17 expression in the human and mouse CNS.
a. UMAP of scRNAseq data of the human brain from Human Protein Atlas. Cells with CXCL17 expression are denoted in black. b. Violin plots illustrating CXCL17 expression by subsets in the hippocampus from healthy donors (n = 2). c. Violin plots of Cxcl17 expression by CNS cells from whole brains of mice at 4-week (n = 2) or 90-week (n = 2). d. Violin plots of Cxcl17 in mouse spinal cord subsets in injury models (n = 3). In B-D mean imputed expression values from individual donors (open circles) and mean values of the donor means (solid circles) are shown with SEM. Sample source information is provided in Supplementary Table 1.
Extended Data Fig. 8 CXCL17 expression in the airway and the gut.
a. Violin plots showing CXCL17 expression in airway epithelial populations of healthy donors and COVID-19 patients. *: p-value < 0.05; **: p-value < 0.01; ***: p-value < 0.001, multivariate regression between healthy and severe COVID-19 samples. b. Violin plots illustrating low expression level of CXCL17 in the gut of healthy donors. Selective expression of GPR15LG in colon and CCL25 in SI are shown for comparison. Mean imputed expression values from individual donors (open circles) and mean values of the donor means (solid circles) are shown with SEM. Sample source information is provided in Supplementary Table 1.
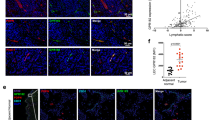
Extended Data Fig. 9 CXCL17 immunohistology of the human cerebellum.
a. CXCL17 immunoreactivity highlights granule neurons (g). b. Reactivity of Purkinje (P) neurons and white matter (wm) surrounding a vessel (v). Methods: Sections of formalin fixed paraffin embedded normal human cerebellum were processed for antigen retrieval and staining with monoclonal mouse IgG anti-human CXCL17 (clone 422204, R&D) using the polymerized goat anti mouse IgG ImmPRESS (peroxidase) kit. DAB shown without counterstain. Isotype control (clone G3A1 mouse IgG1, Cell Signaling) is shown as inset in A. Results representative of 3 or more sections from 2 independent donors.
Supplementary information
Supplementary Fig. 1
Flow cytometry gating strategies for immune cell subsets analysed in this study.
Supplementary Table 1
Detailed sample information of all sequencing data used in this study.
Supplementary Table 2
Molecular weight and pI of human secreted proteins in Fig. 3c.
Supplementary Table 3
Mean BLAST bit-scores from pairwise alignments of the C-terminal six amino acids of each human protein in Fig. 3d and its orthologs in mouse, rat, rabbit, dog and cow.
Supplementary Table 4
Pearson correlation between GPR25 expression and genes in Fig. 3e across 49 non-lymphoid tissues.
Supplementary Table 5
Sequences of CXCL17 variants used in Fig. 3g–i.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ocón, B., Xiang, M., Bi, Y. et al. A lymphocyte chemoaffinity axis for lung, non-intestinal mucosae and CNS. Nature 635, 736–745 (2024). https://doi.org/10.1038/s41586-024-08043-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08043-2