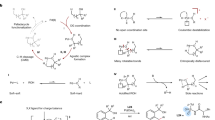
Abstract
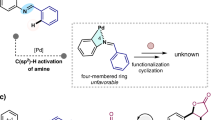
C–H activation is the most direct way of functionalizing organic molecules. Many advances in this field still require specific directing groups to achieve the necessary activity and selectivity. Developing C–H activation reactions directed by native functional groups is essential for their broad application in synthesis1. Over the past decade, several generations of bifunctional ligands developed have enabled C(sp3)–H activation reactions of free carboxylic acids2, free aliphatic amines3, native amides4,5 and alcohols6. However, an effective catalyst for ketones and carboxylic esters remains to be realized. Here we report diverse methyl β-C−H functionalizations, including intermolecular arylation, hydroxylation and intramolecular C(sp3)–H/C(sp2)–H coupling of ketones and carboxylic esters with a monoprotected amino neutral amide (MPANA) ligand. The in situ generation of cationic Pd(II) complexes by the combination MPANA ligand and HBF4 is crucial for achieving the reactivity. The compatibility of these reactions with cyclic ketones and lactams provides a method to access spirocyclic and fused ring systems. Mechanistic experiments and density functional theory studies support the role of cationic Pd complexes with MPANA ligands in enhancing catalyst–substrate affinity and facilitating the C−H cleavage step.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for compound 7eb are available in Supplementary Information files and from the Cambridge Crystallographic Data Center under reference numbers CCDC 2355481. All other data supporting the findings of this study are available in the Article and Supplementary Information.
References
Engle, K. M., Wu, H.-C. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012).
Giri, R. et al. Palladium-catalyzed methylation and arylation of sp2 and sp3 C–H bonds in simple carboxylic acids. J. Am. Chem. Soc. 129, 3510–3511 (2007).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Zhao, R. & Lu, W. Palladium-catalyzed β-arylation of amide via primary sp3 C–H activation. Organometallics 37, 2188–2192 (2018).
Park, H., Chekshin, N., Shen, P.-X. & Yu, J.-Q. Ligand-enabled, palladium-catalyzed β-C(sp3)–H arylation of Weinreb amides. ACS Catal. 8, 9292–9297 (2018).
Strassfeld, D. A. et al. Hydrogen-bond-acceptor ligands enable distal C(sp3)–H arylation of free alcohols. Nature 622, 80–86 (2023).
Mukaiyama, T. Explorations into new reaction chemistry. Angew. Chem. Int. Ed. 43, 5590–5614 (2004).
Evans, D. A., Vogel, E. & Nelson, J. V. Stereoselective aldol condensations via boron enolates. J. Am. Chem. Soc. 101, 6120–6123 (1979).
Myers, A. G. et al. Pseudoephedrine as a practical chiral auxiliary for the synthesis of highly enantiomerically enriched carboxylic acids, alcohols, aldehydes, and ketones. J. Am. Chem. Soc. 119, 6496–6511 (1997).
Constable, A. G., Mcdonald, W. S., Sawkins, L. C. & Shaw, B. L. Palladation of dimethylhydrazones, oximes, and oxime O-allyl ethers: crystal structure of [Pd3(ON = CPriPh)6]. J. Chem. Soc. Chem. Commun. 1978, 1061–1062 (1978).
Desai, L. V., Hull, K. L. & Sanford, M. S. Palladium-catalyzed oxygenation of unactivated sp3 C−H bonds. J. Am. Chem. Soc. 126, 9542–9543 (2004).
Thu, H. Y., Yu, W. Y. & Che, C. M. Intermolecular amidation of unactivated sp2 and sp3 C–H bonds via palladium-catalyzed cascade C–H activation/nitrene insertion. J. Am. Chem. Soc. 128, 9048–9049 (2006).
Zhu, R. Y., Liu, L. Y. & Yu, J. Q. Highly versatile β-C(sp3)-H iodination of ketones using a practical auxiliary. J. Am. Chem. Soc. 139, 12394–12397 (2017).
Zhu, R.-Y. et al. Versatile alkylation of (hetero)aryl iodides with ketones via β-C(sp3)–H activation. J. Am. Chem. Soc. 139, 16080–16083 (2017).
Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp3)−H bonds using a transient directing group. Science 351, 252–256 (2016).
Hong, K., Park, H. & Yu, J.-Q. Methylene C(sp3)–H arylation of aliphatic ketones using a transient directing group. ACS Catal. 7, 6938–6941 (2017).
Pan, L., Yang, K., Li, G. & Ge, H. Palladium-catalyzed site-selective arylation of aliphatic ketones enabled by a transient ligand. Chem. Commun. 54, 2759–2762 (2018).
Li, Y.-H., Ouyang, Y. X., Chekshin, N. & Yu, J.-Q. Pd-II-catalyzed-C(sp3)–H (hetero) arylation of ketones enabled by transient directing groups. ACS Catal. 12, 10581–10586 (2022).
St John-Campbell, S., White, A. J. P. & Bull, J. A. Single operation palladium catalysed C(sp3)–H functionalisation of tertiary aldehydes: investigations into transient imine directing groups. Chem. Sci. 8, 4840–4847 (2017).
Li, Y.-H., Ouyang, Y., Chekshin, N. & Yu, J.-Q. PdII–catalyzed site-selective β- and γ-C(sp3)–H arylation of primary aldehydes controlled by transient directing groups. J. Am. Chem. Soc. 144, 4727–4733 (2022).
He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2017).
Liu, B., Romine, A. M., Rubel, C. Z., Engle, K. M. & Shi, B.-F. Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. Chem. Rev. 121, 14957–15074 (2021).
Shabashov, D. & Daugulis, O. Catalytic coupling of C–H and C–I bonds using pyridine as a directing group. Org. Lett. 7, 3657–3659 (2005).
Alvarez, S. Coordinating ability of anions, solvents, amino acids, and gases towards alkaline and alkaline-earth elements, transition metals, and lanthanides. Chem. Eur. J. 26, 4350–4377 (2020).
Christian, L. & Gal, J.-F. Lewis Basicity and Affinity Scales: Data and Measurement (Wiley, 2010).
Wu, Q.-F. et al. Formation of α-chiral centers by asymmetric β-C(sp3)–H arylation, alkenylation, and alkynylation. Science 355, 499–503 (2017).
Shen, P.-X., Hu, L., Shao, Q., Hong, K. & Yu, J.-Q. Pd(II)-catalyzed enantioselective C(sp3)–H arylation of free carboxylic acids. J. Am. Chem. Soc. 140, 6545–6549 (2018).
Chen, G. et al. Ligand-accelerated enantioselective methylene C(sp3)–H bond activation. Science 353, 1023–1027 (2016).
Zhuang, Z. et al. Ligand-enabled β-C(sp3)–H olefination of free carboxylic acids. J. Am. Chem. Soc. 140, 10363–10367 (2018).
Ng, P. et al. Preparation of 3-spiro-7-hydroxamic acid tetralins as histone deacetylase inhibitors. Patent WO 2016/168660 A1 (2016).
Li, Z. & Yu, J.-Q. Ligand-enabled γ-C(sp3)–H hydroxylation of free amines with aqueous hydrogen peroxide. J. Am. Chem. Soc. 145, 25948–25953 (2023).
Acknowledgements
We thank the Scripps Research Institute and the NIH (National Institute of General Medical Sciences grants 2R01GM084019). We also thank D. A. Strassfeld for assistance with automated conformational searches and discussions throughout this project. We thank J. Chen, B. Sanchez, Q. N. Wong and J. Lee of the Scripps Automated Synthesis Facility for assistance with mass spectrometry. We also thank M. Gembicky and J. Bailey of the UCSD Crystallography Facility for X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
J.-Q.Y. conceived the project. Y.-H.L. developed the MPANA ligand, reaction conditions and substrates scope. N.C. and Y.L. performed the DFT studies. J.-Q.Y. and Y.-H.L. prepared the paper. J.-Q.Y. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
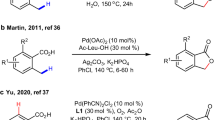
Extended Data Fig. 1 Ineffective ligands for intermolecular methyl β-C–H arylation.
Reaction conditions: substrate (0.1 mmol), Pd(OAc)2 (10 mol%), with or without ligand (10 mol%), 4-iodotoluene (2.0 equiv.), Ag2CO3 (2.0 equiv.), Et2O·HBF4 (1.5 equiv.), HFIP (2.0 ml), 80 °C, 12 h.
Extended Data Fig. 2 Mechanistic investigations.
A, Deuterium incorporation experiments condition: 1a (0.1 mmol), Pd(OAc)2 (10 mol%), ArI (2.0 equiv.), ligand L1 (10 mol%), Ag2CO3 (2.0 equiv.), DOTf (1.5 equiv.), HFIP-OD (2.0 ml), 80 °C, 12 h. B, Parallel kinetic isotopic effect (KIE) studies condition: 1f or [D3]1f (0.1 mmol), Pd(OAc)2 (10 mol%), ArI (2.0 equiv.), ligand L1 (10 mol%), Ag2CO3 (2.0 equiv.), Et2O·HBF4 (1.5 equiv.), HFIP (2.0 ml), 80 °C, 3-75 min. C, Palladium charge and ligand effects on the C(sp3)−H cleavage step. i) Effect of cationic Pd on substrate coordination. ii) Ligand effect on C(sp3)−H cleavage transition state (TS). All quasi-harmonic free energies are reported at 353 K relative to separated reactants. D, C(sp3)−H hydroxylation with water as a solo hydroxyl source. Reaction conditions: 1s (0.1 mmol), PdCl2(PPh3)2 (10 mol%), ligand L3 (10 mol%), Selectfluor (2.5 equiv.), Ag2CO3 (1.5 equiv.), Et2O·HBF4 (1.5 equiv.), water (2.0 equiv.), HFIP (2.0 ml), 50 °C, 48 h, under N2.
Supplementary information
Supplementary Information
Supplementary Sections 1–15; see Contents for details.
Crystallographic Data (7b)
Crystallographic data for compound 7b
Crystallographic Data (7eb)
Crystallographic data for compound 7eb
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YH., Chekshin, N., Lu, Y. et al. β-C−H bond functionalization of ketones and esters by cationic Pd complexes. Nature 637, 608–614 (2025). https://doi.org/10.1038/s41586-024-08281-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08281-4