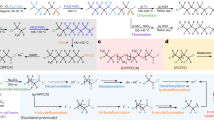
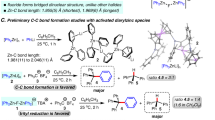
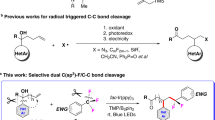
Abstract
Organic halides are highly useful compounds in chemical synthesis, in which the halide serves as a versatile functional group for elimination, substitution and cross-coupling reactions with transition metals or photocatalysis1,2,3. However, the activation of carbon–fluorine (C–F) bonds—the most commercially abundant organohalide and found in polyfluoroalkyl substances (PFAS), or ‘forever chemicals’—is much rarer. Current approaches based on photoredox chemistry for the activation of small-molecule C–F bonds are limited by the substrates and transition metal catalysts needed4. A general method for the direct activation of organofluorines would have considerable value in organic and environmental chemistry. Here we report an organic photoredox catalyst system that can efficiently reduce C–F bonds to generate carbon-centred radicals, which can then be intercepted for hydrodefluorination (swapping F for H) and cross-coupling reactions. This system enables the general use of organofluorines as synthons under mild reaction conditions. We extend this method to the defluorination of PFAS and fluorinated polymers, a critical challenge in the breakdown of persistent and environmentally damaging forever chemicals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Materials and methods, experimental procedures, useful information, mechanistic studies, 1H NMR spectra, 13C NMR spectra, 18F NMR, gel permeation chromatography, mass spectrometry data and DFT calculation results are available in the Supplementary Information.
References
Cheung, K. P. S., Sarkar, S. & Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev. 122, 1543–1625 (2022).
Pitre, S. P. & Overman, L. E. Strategic use of visible-light photoredox catalysis in natural product synthesis. Chem. Rev. 122, 1717–1751 (2022).
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011).
Hooker, L. V. & Bandar, J. S. Synthetic advantages of defluorinative C−F bond functionalization. Angew. Chem. Int. Ed. 135, e202308880 (2023).
Wang, J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506 (2014).
Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 5, 570–589 (2004).
Pagliaro, M. & Ciriminna, R. New fluorinated functional materials. J. Mater. Chem. 15, 4981–4991 (2005).
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Puts, G. J., Crouse, P. & Ameduri, B. M. Polytetrafluoroethylene: synthesis and characterization of the original extreme polymer. Chem. Rev. 119, 1763–1805 (2019).
Améduri, B. The promising future of fluoropolymers. Macromol. Chem. Phys. 221, 1900573 (2020).
Furuya, T., Kamlet, A. S. & Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477 (2011).
Amii, H. & Uneyama, K. C−F bond activation in organic synthesis. Chem. Rev. 109, 2119–2183 (2009).
Joudan, S. & Lundgren, R. J. Taking the ‘F’ out of forever chemicals. Science 377, 816–817 (2022).
Stahl, T., Klare, H. F. T. & Oestreich, M. Main-group Lewis acids for C–F bond activation. ACS Catal. 3, 1578–1587 (2013).
Aizenberg, M. & Milstein, D. Catalytic activation of carbon-fluorine bonds by a soluble transition metal complex. Science 265, 359–361 (1994).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Yoon, T. P., Ischay, M. A. & Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2, 527–532 (2010).
Yan, G. Photochemical and electrochemical strategies for hydrodefluorination of fluorinated organic compounds. Chem. Eur. J. 28, e202200231 (2022).
Wang, Z. et al. Photochemical and electrochemical strategies in C–F bond activation and functionalization. Org. Chem. Front. 9, 853–873 (2022).
Theriot, J. C. et al. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 352, 1082–1086 (2016).
Ghosh, I., Ghosh, T., Bardagi, J. I. & König, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 346, 725–728 (2014).
MacKenzie, I. A. et al. Discovery and characterization of an acridine radical photoreductant. Nature 580, 76–90 (2020).
Kerzig, C., Guo, X. & Wenger, O. S. Unexpected hydrated electron source for preparative visible-light driven photoredox catalysis. J. Am. Chem. Soc. 141, 2122–2127 (2019).
Rieth, A. L., Gonzalez, M. I., Kudisch, B., Nava, M. & Nocera, D. G. How radical are ‘radical’ photocatalysts? A closed-shell Meisenheimer complex is identified as a super-reducing photoreagent. J. Am. Chem. Soc. 143, 14352–14359 (2021).
Cowper, N. G. W., Chernowsky, C. P., Williams, O. P. & Wickens, Z. K. Potent reductants via electron-primed photoredox catalysis: unlocking aryl chlorides for radical coupling. J. Am. Chem. Soc. 142, 2093–2099 (2020).
Cole, J. P. et al. Organocatalyzed Birch reduction driven by visible light. J. Am. Chem. Soc. 142, 13573–13581 (2020).
Saha, S. Anion-induced electron transfer. Acc. Chem. Res. 51, 2225–2236 (2018).
Sau, A. et al. Mechanistic investigation of a photocatalyst model reveals function by perylene-like closed shell super-photoreductant capable of reducing unactivated arenes. ACS Catal. 14, 2252–2263 (2024).
Han, J. et al. Using preformed Meisenheimer complexes as dopants for n-type organic thermoelectrics with high Seebeck coefficients and power factors. Adv. Funct. Mater. 31, 2010567 (2021).
Dawson, R. E. et al. Experimental evidence for the functional relevance of anion–π interactions. Nat. Chem. 2, 533–538 (2010).
Aragay, G., Frontera, A., Llovera, V., Vidal-Gancedo, J. & Ballester Different nature of the interactions between anions and HAT(CN)6: from reversible anion−π complexes to irreversible electron-transfer processes (HAT(CN)6 = 1,4,5,8,9,12-hexaazatriphenylene). J. Am. Chem. Soc. 135, 2620–2627 (2013).
Glaser, F., Kerzig, C. & Wenger, O. S. Multi-photon excitation in photoredox catalysis: concepts, applications, methods. Angew. Chem. Int. Ed. 59, 10266–10284 (2020).
Trojanowicz, M., Bojanowska-Czajka, A., Bartosiewicz, I. & Kulisa, K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—a review of recent advances. Chem. Eng. J. 336, 170–199 (2018).
Longendyke, G. K., Katel, S. & Wang, Y. PFAS fate and destruction mechanisms during thermal treatment: a comprehensive review. Environ. Sci.: Processes Impacts 24, 196–208 (2022).
Douvris, C. & Ozerov, O. V. Hydrodefluorination of perfluoroalkyl groups using silylium-carborane catalysts. Science 321, 1188–1190 (2008).
Lohmann, R. et al. Are fluoropolymers really of low concern for human and environmental health and separate from other PFAS? Environ. Sci. Technol. 54, 12820–12828 (2020).
Acknowledgements
X.L., A.R.G., N.F.P., A.S., N.H.D. and G.M.M. acknowledge support from the National Science Foundation (2016557). Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM144356. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.S.P., M.V.P. and Y.L. acknowledge support from the NSF (CHE-1955876) and computational resources from the RMACC Summit supercomputer supported by the NSF (grant nos. ACI-1532235 and ACI-1532236), the University of Colorado Boulder and Colorado State University, and XSEDE through allocation no. TG-CHE180056.
Author information
Authors and Affiliations
Contributions
X.L. and G.M.M. conceived the idea. X.L., A.R.G. and Y.Z. executed the reaction methodology development. N.F.P., A.S., M.V.P., N.H.D., X.L. and A.R.G. conceived of and contributed to the mechanistic studies. M.V.P., Y.L. and R.S.P. performed the DFT calculations. X.L., N.F.P., A.S., R.S.P., N.H.D. and G.M.M. co-wrote the manuscript. All authors read and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jinyong Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Formation of BPI-N-RO− from BPI-N.
a, Using a more symmetric variant BPI-N for intermediate study. b, Comparing the UV–visible spectra of isolated BPI-N-RO− (post basic alumina column with and without aqueous workup) with that of the crude BPI-N with nBu4NF mixture. Absorption spectrum of BPI-N is shown for reference. c, 1H-1H COSY of the isolated intermediate BPI-N-RO− (acetone-d6, 300 MHz). d, 1H-13C HSQC of the isolated intermediate BPI-N-RO− (acetone-d6, 300 MHz).
Extended Data Fig. 2 Cyclic voltammetry of BPI and BPI with nBu4NF.
a, Cyclic voltammogram of Fc+/Fc standard (1.0 mM in THF). b, Cyclic voltammogram of BPI (1.0 mM in THF) showing the first reduction event. c, Cyclic voltammogram of BPI (1.0 mM in THF) showing two reduction events. d, Cyclic voltammogram of BPI (1.0 mM in THF) with nBu4NF (200 equiv.) showing two reduction events.
Extended Data Fig. 3 Mechanistic studies.
a, Emission profiles for BPI and BPI-RO–. b, Fluorescence spectra of BPI in a THF solution in the presence of a varying equivalence of nBu4NF. c, Monitoring BPI and nBu4NF interaction via UV–vis spectroscopy. d, Quenching experiments on BPI-RO2−• by Ph-F. e, Quenching experiments on BPI-RO-H2− by Ph-F. f, Quenching experiments on BPI-RO-H2− by methyl 4-flurobenzoate.
Extended Data Fig. 4 Computed reduction potentials for the proposed photocatalytic species.
Using the CAM-B3LYP-D3(BJ)/def2-SVP[C;H]def2-TZVPD[N;O;F]-SMD(THF) level of theory.
Extended Data Fig. 5 Computed hydrolysis mechanisms.
The model system C at the M06-2X-D3/def2-SVP[C;H]def2-TZVPD[N;O;F]-SMD(THF) was used.
Extended Data Fig. 6 Proposed structures of the ring-opened intermediate and closed shell species.
Original catalyst (left), isolated ring-opening intermediate (middle), suggested closed-shell species (right).
Supplementary information
Supplementary Information
Supplementary Information sections 1–15, Figs. 1–66, Tables 1–22 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Sau, A., Green, A.R. et al. Photocatalytic C–F bond activation in small molecules and polyfluoroalkyl substances. Nature 637, 601–607 (2025). https://doi.org/10.1038/s41586-024-08327-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08327-7
This article is cited by
-
How to get rid of toxic ‘forever chemical’ pollution
Nature (2025)
-
Phosphate-enabled mechanochemical PFAS destruction for fluoride reuse
Nature (2025)
-
‘Forever’ chemicals can be destroyed with clever chemistry — now test these techniques outside the lab
Nature (2024)
-
Catalysts degrade forever chemicals with visible light
Nature (2024)