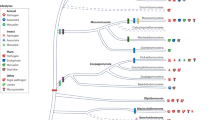
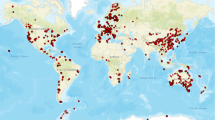
Abstract
Over the past billion years, the fungal kingdom has diversified to more than two million species, with over 95% still undescribed. Beyond the well-known macroscopic mushrooms and microscopic yeast, fungi are heterotrophs that feed on almost any organic carbon, recycling nutrients through the decay of dead plants and animals and sequestering carbon into Earth’s ecosystems. Human-directed applications of fungi extend from leavened bread, alcoholic beverages and biofuels to pharmaceuticals, including antibiotics and psychoactive compounds. Conversely, fungal infections pose risks to ecosystems ranging from crops to wildlife to humans; these risks are driven, in part, by human and animal movement, and might be accelerating with climate change. Genomic surveys are expanding our knowledge of the true biodiversity of the fungal kingdom, and genome-editing tools make it possible to imagine harnessing these organisms to fuel the bioeconomy. Here, we examine the fungal threats facing civilization and investigate opportunities to use fungi to combat these threats.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hawksworth, D. L. & Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 5, FUNK-0052-2016 (2017).
Baldrian, P., Kohout, P. & Větrovský, T. in Evolution of Fungi and Fungal-Like Organisms (eds. Pöggeler, S. & James, T.) 227–238 (Springer, 2023).
Niskanen, T. et al. Pushing the frontiers of biodiversity research: unveiling the global diversity, distribution, and conservation of fungi. Annu. Rev. Environ. Resour. 48, 149–176 (2023).
Brundrett, M. C. & Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115 (2018).
Bergman, A. & Casadevall, A. Mammalian endothermy optimally restricts fungi and metabolic costs. mBio 1, e00212-10 (2010).
Denning, D. W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24, e428–e438 (2024).
Lilleskov, E. A., Kuyper, T. W., Bidartondo, M. I. & Hobbie, E. A. in Atmospheric Nitrogen Deposition to Global Forests (eds. Du, E. & de Vries, W.) 95–118 (Academic, 2024).
Raza, M. M. & Bebber, D. P. Climate change and plant pathogens. Curr. Opin. Microbiol. 70, 102233 (2022).
Head, J. R. et al. Effects of precipitation, heat, and drought on incidence and expansion of coccidioidomycosis in western USA: a longitudinal surveillance study. Lancet Planet. Health 6, e793–e803 (2022).
Steidinger, B. S. et al. Ectomycorrhizal fungal diversity predicted to substantially decline due to climate changes in North American Pinaceae forests. J. Biogeogr. 47, 772–782 (2020).
Qin, C., Pellitier, P. T., Van Nuland, M. E., Peay, K. G. & Zhu, K. Niche modelling predicts that soil fungi occupy a precarious climate in boreal forests. Glob. Ecol. Biogeogr. 32, 1127–1139 (2023).
Van Nuland, M. E. et al. Above- and belowground fungal biodiversity of Populus trees on a continental scale. Nat. Microbiol. 8, 2406–2419 (2023).
Robert, V. A. & Casadevall, A. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 200, 1623–1626 (2009).
Seidel, D. et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe 5, e594–e605 (2024).
Garcia-Solache, M. A. & Casadevall, A. Global warming will bring new fungal diseases for mammals. mBio 1, e00061-10 (2010).
Salthammer, T. et al. A holistic modeling framework for estimating the influence of climate change on indoor air quality. Indoor Air 32, e13039 (2022).
Awaab Ishak and the politics of mould in the UK. eClinicalMedicine 54, 101801 (2022).
Li, Y. et al. Impact of Hurricane Harvey on inpatient asthma hospitalization visits within southeast Texas, 2016–2019. J. Occup. Environ. Med. 65, 924–930 (2023).
Toda, M. et al. Invasive mold infections following Hurricane Harvey—Houston, Texas. Open Forum Infect. Dis. 10, ofad093 (2023).
Mulchandani, R. et al. The English National Cohort Study of Flooding & Health: psychological morbidity at three years of follow up. BMC Public Health 20, 321 (2020).
Doehlemann, G., Ökmen, B., Zhu, W. & Sharon, A. Plant pathogenic fungi. Microbiol. Spectr. 5, FUNK-0023-2016 (2017).
Stukenbrock, E. & Gurr, S. Address the growing urgency of fungal disease in crops. Nature 617, 31–34 (2023).
Fisher, M. C., Hawkins, N. J., Sanglard, D. & Gurr, S. J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360, 739–742 (2018).
Robey, M. T., Caesar, L. K., Drott, M. T., Keller, N. P. & Kelleher, N. L. An interpreted atlas of biosynthetic gene clusters from 1,000 fungal genomes. Proc. Natl Acad. Sci. USA 118, e2020230118 (2021). Comprehensive characterization of the biosynthetic potential of fungi to produce natural products.
Ekanayaka, A. H. et al. A review of the fungi that degrade plastic. J. Fungi 8, 772 (2022).
Schmidt, B. et al. Mechanical, physical and thermal properties of composite materials produced with the basidiomycete Fomes fomentarius. Fungal Biol. Biotechnol. 10, 22 (2023).
Cordero, R. J. B. & Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 31, 99–112 (2017).
Dadachova, E. et al. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2, e457 (2007).
Berbee, M. L., James, T. Y. & Strullu-Derrien, C. Early diverging fungi: diversity and impact at the dawn of terrestrial life. Annu. Rev. Microbiol. 71, 41–60 (2017).
Amend, A. et al. Fungi in the marine environment: open questions and unsolved problems. mBio 10, e01189-18 (2019).
Geiser, D. M., Taylor, J. W., Ritchie, K. B. & Smith, G. W. Cause of sea fan death in the West Indies. Nature 394, 137–138 (1998).
Rosenberg, J. F. et al. Cryptococcus gattii type VGIIa infection in harbor seals (Phoca vitulina) in British Columbia, Canada. J. Wildl. Dis. 52, 677–681 (2016).
Harris, H. S. et al. Novel presentation of coccidioidomycosis with myriad free-floating proteinaceous spheres in the pericardial sac of a southern sea otter (Enhydra lutris nereis). J. Wildl. Dis. 60, 223–228 (2024).
Vilela, R. & Mendoza, L. in Emerging and Epizootic Fungal Infections in Animals (eds. Seyedmousavi, S. et al.) 177–196 (Springer, 2018).
Schmidt, S., Kildgaard, S., Guo, H., Beemelmanns, C. & Poulsen, M. The chemical ecology of the fungus-farming termite symbiosis. Nat. Prod. Rep. 39, 231–248 (2022).
Currie, C. R., Poulsen, M., Mendenhall, J., Boomsma, J. J. & Billen, J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311, 81–83 (2006). The authors discover modifications in fungus-growing ants for maintaining bacterial symbionts that produce antimicrobials to suppress fungal pathogens, illustrating the complexity of fungus–insect interactions.
Boyce, G. R. et al. Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying cicada pathogens. Fungal Ecol. 41, 147–164 (2019).
Raffa, K. F. et al. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58, 501–517 (2008).
Kandasamy, D. et al. Conifer-killing bark beetles locate fungal symbionts by detecting volatile fungal metabolites of host tree resin monoterpenes. PLoS Biol. 21, e3001887 (2023).
Scheele, B. C. et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463 (2019). Analysis showing the extent to which the amphibian chytridiomycosis panzootic has caused the greatest recorded loss of biodiversity attributable to a disease.
Hoyt, J. R., Kilpatrick, A. M. & Langwig, K. E. Ecology and impacts of white-nose syndrome on bats. Nat. Rev. Microbiol. 19, 196–210 (2021). Summarizes the origin and introduction of Pseudogymnoascus destructans, the fungal pathogen that causes bat white-nose syndrome, to North America and describes the impacts and epidemiology of this devastating wildlife disease.
Coker, T. L. R. et al. Estimating mortality rates of European ash (Fraxinus excelsior) under the ash dieback (Hymenoscyphus fraxineus) epidemic. Plants People Planet 1, 48–58 (2019).
O’Hanlon, S. J. et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360, 621–627 (2018). Genomic analysis identifying the centre of origin and dating the worldwide expansion of the amphibian chytridiomycosis panzootic.
Martel, A. et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl Acad. Sci. USA 110, 15325–15329 (2013).
Frick, W. F. et al. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682 (2010).
McMullan, M. et al. The ash dieback invasion of Europe was founded by two genetically divergent individuals. Nat. Ecol. Evol. 2, 1000–1008 (2018).
Fisher, M. C. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194 (2012).
Springborn, M. R., Weill, J. A., Lips, K. R., Ibáñez, R. & Ghosh, A. Amphibian collapses increased malaria incidence in Central America. Environ. Res. Lett. 17, 104012 (2022).
Boyles, J. G., Cryan, P. M., McCracken, G. F. & Kunz, T. H. Economic importance of bats in agriculture. Science 332, 41–42 (2011).
Hill, L. et al. The £15 billion cost of ash dieback in Britain. Curr. Biol. 29, R315–R316 (2019).
Taylor, J. W. & Barker, B. M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 57, S16–S20 (2019).
Gorris, M. E., Neumann, J. E., Kinney, P. L., Sheahan, M. & Sarofim, M. C. Economic valuation of coccidioidomycosis (valley fever) projections in the United States in response to climate change. Weather Clim. Soc. 13, 107–123 (2021).
Gorris, M. E., Treseder, K. K., Zender, C. S. & Randerson, J. T. Expansion of coccidioidomycosis endemic regions in the United States in response to climate change. GeoHealth 3, 308–327 (2019).
Rocke, T. E. et al. Virally-vectored vaccine candidates against white-nose syndrome induce anti-fungal immune response in little brown bats (Myotis lucifugus). Sci. Rep. 9, 6788 (2019).
Isidoro-Ayza, M. & Klein, B. S. Pathogenic strategies of Pseudogymnoascus destructans during torpor and arousal of hibernating bats. Science 385, 194–200 (2024).
Waddle, A. W. et al. Hotspot shelters stimulate frog resistance to chytridiomycosis. Nature 631, 344–349 (2024).
Heiniger, U. & Rigling, D. Biological control of chestnut blight in Europe. Annu. Rev. Phytopathol. 32, 581–599 (1994).
Zhang, H. et al. A 2-kb mycovirus converts a pathogenic fungus into a beneficial endophyte for Brassica protection and yield enhancement. Mol. Plant 13, 1420–1433 (2020).
Thapa, V., Keller, N. P. & Roossinck, M. J. Evaluation of virus-free and wild-type isolates of Pseudogymnoascus destructans using a porcine ear model. mSphere 7, P1420–P1433 (2022).
Clemons, R. A. et al. An endogenous DNA virus in an amphibian-killing fungus associated with pathogen genotype and virulence. Curr. Biol. 34, 1469–1478 (2024).
Feijen, F. A. A., Vos, R. A., Nuytinck, J. & Merckx, V. S. F. T. Evolutionary dynamics of mycorrhizal symbiosis in land plant diversification. Sci. Rep. 8, 10698 (2018).
Busby, P. E. et al. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 15, e2001793 (2017).
Allsup, C. M., George, I. & Lankau, R. A. Shifting microbial communities can enhance tree tolerance to changing climates. Science 380, 835–840 (2023).
Hawkes, C. V., Allen, X., Balint-Kurti, P. & Cowger, C. Manipulating the plant mycobiome to enhance resilience: Ecological and evolutionary opportunities and challenges. PLoS Pathog. 19, e1011816 (2023).
Chitnis, V. R. et al. Fungal endophyte-mediated crop improvement: the way ahead. Front. Plant Sci. 11, 561007 (2020).
Morales-Vargas, A. T., López-Ramírez, V., Álvarez-Mejía, C. & Vázquez-Martínez, J. Endophytic fungi for crops adaptation to abiotic stresses. Microorganisms 12, 1357 (2024).
Gowtham, H. G. et al. Fungal endophytes as mitigators against biotic and abiotic stresses in crop plants. J. Fungi 10, 116 (2024).
Hawkins, H. J. et al. Mycorrhizal mycelium as a global carbon pool. Curr. Biol. 33, R560–R573 (2023). The first global quantification of carbon allocation from plants to mycorrhizal fungi estimates that 13.12 Gt of CO2 emissions fixed by terrestrial plants is, at least temporarily, allocated to the underground mycelium of mycorrhizal fungi per year, equating to around 36% of current annual CO2 emissions from fossil fuels.
Lilleskov, E. A., Kuyper, T. W., Bidartondo, M. I. & Hobbie, E. A. Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review. Environ. Pollut. 246, 148–162 (2019).
Stukenbrock, E. H. et al. The making of a new pathogen: insights from comparative population genomics of the domesticated wheat pathogen Mycosphaerella graminicola and its wild sister species. Genome Res. 21, 2157–2166 (2011).
Sotiropoulos, A. G. et al. Global genomic analyses of wheat powdery mildew reveal association of pathogen spread with historical human migration and trade. Nat. Commun. 13, 4315 (2022). This study traced the historical spread of wheat powdery mildew, revealing the role of human migration as well as rapid evolution through hybridization.
Fones, H. N. et al. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 1, 332–342 (2020).
Sharma, R. R., Singh, D. & Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol. Control 50, 205–221 (2009).
Singh, R. P. et al. Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology 105, 872–884 (2015).
Grandaubert, J., Dutheil, J. Y. & Stukenbrock, E. H. The genomic determinants of adaptive evolution in a fungal pathogen. Evol. Lett. 3, 299–312 (2019).
Islam, M. T. et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 14, 84 (2016).
Eskola, M. et al. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 60, 2773–2789 (2020).
Liew, W. P. P. & Mohd-Redzwan, S. Mycotoxin: its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 8, 60 (2018).
Johns, L. E., Bebber, D. P., Gurr, S. J. & Brown, N. A. Emerging health threat and cost of Fusarium mycotoxins in European wheat. Nat. Food 3, 1014–1019 (2022).
Sun, Y., Song, Y., Long, M. & Yang, S. Immunotoxicity of three environmental mycotoxins and their risks of increasing pathogen infections. Toxins 15, 187 (2023).
Wang, Y., Pruitt, R. N., Nürnberger, T. & Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 20, 449–464 (2022).
Weiberg, A. et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342, 118–123 (2013). This study discovered the mechanism of cross-kingdom RNA interference (RNAi), in which small RNAs from a fungal pathogen are transported into plant hosts and use the plant RNAi machinery to silence plant immunity genes.
Zhang, B., Sen, Li,Y. C., Guo, H. S. & Zhao, J. H. Verticillium dahliae secretes small RNA to target host MIR157d and retard plant floral transition during infection. Front. Plant Sci. 13, 847086 (2022).
Cai, Q. et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360, 1126–1129 (2018).
Chaloner, T. M., Gurr, S. J. & Bebber, D. P. Plant pathogen infection risk tracks global crop yields under climate change. Nat. Clim. Change 11, 710–715 (2021).
Oliver, R. P. & Hewitt, H. G. Fungicides in Crop Protection (CABI, 2014).
Steinberg, G. et al. A lipophilic cation protects crops against fungal pathogens by multiple modes of action. Nat. Commun. 11, 1608 (2020).
Van Den Bosch, F., Paveley, N., Van Den Berg, F., Hobbelen, P. & Oliver, R. Mixtures as a fungicide resistance management tactic. Phytopathology 104, 1264–1273 (2014).
Ludwig, N. et al. A cell surface-exposed protein complex with an essential virulence function in Ustilago maydis. Nat. Microbiol. 6, 722–730 (2021). This study describes a complex of seven Ustilago maydis proteins likely to be involved in effector delivery to the host.
Niu, D. et al. RNAs—a new frontier in crop protection. Curr. Opin. Biotechnol. 70, 204–212 (2021).
Cai, Q. et al. Message in a bubble: shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. Plant Biol. 72, 497–524 (2021).
Qiao, L. et al. Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotechnol. J. 21, 854–865 (2023).
Luo, M. et al. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat. Biotechnol. 39, 561–566 (2021).
Kourelis, J., Marchal, C., Posbeyikian, A., Harant, A. & Kamoun, S. NLR immune receptor-nanobody fusions confer plant disease resistance. Science 379, 934–939 (2023).
Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action (WHO, 2022).
Lionakis, M. S., Drummond, R. A. & Hohl, T. M. Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 23, 433–452 (2023).
Hoenigl, M. et al. COVID-19-associated fungal infections. Nat. Microbiol. 7, 1127–1140 (2022).
Nash, A. K. et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5, 153 (2017).
Leonardi, I. et al. Mucosal fungi promote gut barrier function and social behavior via type 17 immunity. Cell 185, 831–846 (2022).
Li, X. V. et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603, 672–678 (2022).
Martini, G. R. et al. Selection of cross-reactive T cells by commensal and food-derived yeasts drives cytotoxic TH1 cell responses in Crohn’s disease. Nat. Med. 29, 2602–2614 (2023).
Bacher, P. et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell 176, 1340–1355 (2019). This study showed that intestinal Candida albicans is the most common inducer of TH17 responses in the gut of humans, and that these cells are cross-reactive to Aspergillus in the airway causing inflammation. This is suggestive of a gut-to-lung axis in which TH17 immune protective responses in the gut can exacerbate pathological inflammation in the lung.
Shao, T. Y. et al. Commensal Candida albicans positively calibrates systemic Th17 immunological responses. Cell Host Microbe 25, 404–417 (2019).
Bradford, L. L. & Ravel, J. The vaginal mycobiome: a contemporary perspective on fungi in women’s health and diseases. Virulence 8, 342–351 (2017).
Wu, G. et al. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet. 11, e1005614 (2015).
Findley, K. et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370 (2013).
Sparber, F. et al. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25, 389–403 (2019).
Hernández-Santos, N. et al. Lung epithelial cells coordinate innate lymphocytes and immunity against pulmonary fungal infection. Cell Host Microbe 23, 511–522 (2018).
Conti, H. R. et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 20, 606–617 (2016).
Ost, K. S. et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 596, 114–118 (2021).
Doron, I. et al. Mycobiota-induced IgA antibodies regulate fungal commensalism in the gut and are dysregulated in Crohn’s disease. Nat. Microbiol. 6, 1493–1504 (2021).
Doron, I. et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 184, 1017–1031 (2021).
Liang, S. H. et al. The hyphal-specific toxin candidalysin promotes fungal gut commensalism. Nature 627, 620–627 (2024).
Rhodes, J. et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat. Microbiol. 7, 663–674 (2022). Genomic analysis revealing the infection of patients with antifungal resistant isolates acquired from environment exposures.
Jacobs, S. E., Zagaliotis, P. & Walsh, T. J. Novel antifungal agents in clinical trials. F1000Research 10, 507 (2021).
Maji, A. et al. Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature 623, 1079–1085 (2023).
van Rhijn, N. et al. Aspergillus fumigatus strains that evolve resistance to the agrochemical fungicide ipflufenoquin in vitro are also resistant to olorofim. Nat. Microbiol. 9, 29–34 (2023).
Break, T. J. et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 371, eaay5731 (2021).
Oliveira, L. V. N., Wang, R., Specht, C. A. & Levitz, S. M. Vaccines for human fungal diseases: close but still a long way to go. NPJ Vaccines 6, 33 (2021).
Rayens, E. et al. Immunogenicity and protective efficacy of a pan-fungal vaccine in preclinical models of aspergillosis, candidiasis, and pneumocystosis. PNAS Nexus 1, pgac248 (2022).
Lockhart, S. R., Chowdhary, A. & Gold, J. A. W. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 21, 818–832 (2023). This study describes the global population structure of Candida auris and defines the four major clades that arose contemporaneously, revealing the low diversity of isolates within each clade and high diversity between clades. This led to many hypotheses about what could have led to the rapid emergence of this pathogen with its complex population structure.
Duong, T. M. N. et al. Azole-resistant Aspergillus fumigatus is highly prevalent in the environment of Vietnam, with marked variability by land use type. Environ. Microbiol. 23, 7632–7642 (2021).
Chow, N. A. et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 11, e03364-19 (2020).
Arora, P. et al. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. mBio 12, e03181-20 (2021).
Yadav, A. et al. Candida auris on apples: diversity and clinical significance. mBio 13, e0051822 (2022).
Rybak, J. M. et al. Mutations in TAC1B: a novel genetic determinant of clinical fluconazole resistance in Candida auris. mBio 11, e00365-20 (2020).
Lockhart, S. R. et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 64, 134–140 (2017).
Casadevall, A., Kontoyiannis, D. P. & Robert, V. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio 10, e01397-19 (2019).
Jackson, B. R. et al. On the origins of a species: what might explain the rise of Candida auris? J. Fungi 5, 58 (2019).
Uhrlaß, S. et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J. Fungi 8, 757 (2022). Summary of the characteristics of a rapidly emerging epidemic fungus in India, Trichophyton indotineae, that causes a skin-to-skin transmission affecting the groin, body and face. T. indotineae is resistant to terbinafine, which is normally the antifungal of choice for dermatophyte fungi.
Etchecopaz, A. et al. Sporothrix brasiliensis: a review of an emerging South American fungal pathogen, its related disease, presentation and spread in Argentina. J. Fungi 7, 170 (2021).
Barnacle, J. R. et al. The first three reported cases of Sporothrix brasiliensis cat-transmitted sporotrichosis outside South America. Med. Mycol. Case Rep. 39, 14–17 (2022).
Yadav, A. et al. Candida auris in dog ears. J. Fungi 9, 720 (2023).
White, T. C. et al. Candida auris detected in the oral cavity of a dog in Kansas. mBio 15, e0308023 (2024).
Huang, J. et al. Pan-drug resistance and hypervirulence in a human fungal pathogen are enabled by mutagenesis induced by mammalian body temperature. Nat. Microbiol. 9, 1686–1699 (2024).
Graham, A. E. & Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 14, 2231 (2023).
Jahn, L. J., Rekdal, V. M. & Sommer, M. O. A. Microbial foods for improving human and planetary health. Cell 186, 469–478 (2023).
Behera, B. C. Citric acid from Aspergillus niger: a comprehensive overview. Crit. Rev. Microbiol. 46, 727–749 (2020).
Fraser, R. Z., Shitut, M., Agrawal, P., Mendes, O. & Klapholz, S. Safety evaluation of soy leghemoglobin protein preparation derived from Pichia pastoris, intended for use as a flavor catalyst in plant-based meat. Int. J. Toxicol. 37, 241–262 (2018). This study revealed no evidence for toxicity for the haem cofactor from plant leghaemoglobin that confers meat-like flavours upon cooking.
Li, J. et al. Molecular mechanisms of nematode–nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 53, 67–95 (2015).
Yang, X. et al. Cyclosporine biosynthesis in Tolypocladium inflatum benefits fungal adaptation to the environment. mBio 9, e01211-18 (2018).
Sanchez, S. & Demain, A. L. in Food Bioactives (ed. Puri, M.) 59–87 (Springer, 2017).
Nutt, D., Spriggs, M. & Erritzoe, D. Psychedelics therapeutics: what we know, what we think, and what we need to research. Neuropharmacology 223, 109257 (2023).
Lark, T. J. et al. Environmental outcomes of the US Renewable Fuel Standard. Proc. Natl Acad. Sci. USA 119, e2101084119 (2022).
Huq, N. A. et al. Toward net-zero sustainable aviation fuel with wet waste-derived volatile fatty acids. Proc. Natl Acad. Sci. USA 118, e2023008118 (2021).
Harms, H., Schlosser, D. & Wick, L. Y. Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 9, 177–192 (2011).
Vandelook, S., Elsacker, E., Van Wylick, A., De Laet, L. & Peeters, E. Current state and future prospects of pure mycelium materials. Fungal Biol. Biotechnol. 8, 20 (2021).
Cordero, R. J. B., Mattoon, E. R., Ramos, Z. & Casadevall, A. The hypothermic nature of fungi. Proc. Natl Acad. Sci. USA 120, e2221996120 (2023).
Acknowledgements
N.T.C. is supported by a Canadian Institutes for Health Research (CIHR) Canada Graduate Scholarships Doctoral Award. A. Casadevall was supported by National Institutes of Health (NIH) grants AI052733-16, AI152078-01 and HL059842-19. C.R.C. was supported by the Jarislowsky Foundation. L.K.F.-L., T.Y.J. and J.E.S. are supported by the Gordon and Betty Moore Foundation award 9337 (10.37807/GBMF9337). A.G. is supported by an NIH–NIAID K99 grant 1K99AI166094-01. I.D.I. is supported by NIH grants DK113136, DK121977 and AI163007. H.J. was supported by grants from the NIH (R35GM136379), National Science Foundation (NSF) (IOS 2020731), US Department of Agriculture (2021-67013-34258) and Australian Research Council Research Hub for Sustainable Crop Protection (IH190100022). B.S.K. is supported by grants R01 AI168370, R37 AI035681, R01 AI040996, U01 AI124299, NSF 2301729, T32 AI055397 and BAA-NIAID-DAIT-AI201800007. R.S.S. is supported by a Tier II Canada Research Chair. K.G.P. is supported by NSF DEB 1845544 and DOE BER DE-SC0023661. J.E.S. was supported by NSF grant EF-2125066, NIH–NIAID grants AI127548 AI130128 and US Department of Agriculture, National Institute of Food and Agriculture Hatch Projects CA-R-PPA-211-5062-H. L.E.C. is a Canada Research Chair (Tier 1) in Microbial Genomics and Infectious Disease and is supported by a CIHR Foundation grant (FDN-154288) and NIH grants R01 AI127375, R01 AI162789 and R01AI165466. J.H. is supported by NIH grants R01 AI039115, R01 AI050113, R01 AI170543, R01 AI172451, R01 AI133654 and R21 AI168672. J.A.S. is supported by the Intramural Research Program of the National Human Genome Research Institute. I.V.E., A.C.G., A.G., K.S.O., R.S.S. and N.S. are CIFAR Azrieli Global Scholars of the CIFAR Fungal Kingdom: Threats & Opportunities program. S.J.G., M.C.F., D.S.B., C.B., A. Chowdhary, C.A.C., C.R.C., L.K.F.-L., I.D.I., T.Y.J., H.J., B.S.K., J.W.K., K.G.P., D.C.S., J.E.S., E.H.S., G.D.W. and J.A.S. are Fellows of the CIFAR Fungal Kingdom: Threats & Opportunities program. A. Casadevall, D.W.D., N.A.R.G., R.K. and J.W.T. are advisors of the CIFAR Fungal Kingdom: Threats & Opportunities program. J.H. and L.E.C. are co-directors of the CIFAR Fungal Kingdom: Threats & Opportunities program. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US government.
Author information
Authors and Affiliations
Contributions
N.T.C., S.J.G., M.C.F., D.S.B., C.B., A. Casadevall, A. Chowdhary, C.A.C., C.R.C., D.W.D., I.V.E., L.K.F.-L., A.C.G., N.A.R.G., A.G., I.D.I., T.Y.J., H.J., R.K., B.S.K., J.W.K., K.S.O., K.G.P., R.S.S., D.C.S., N.S., J.E.S., E.H.S., J.W.T., G.D.W., L.E.C., J.H. and J.A.S. conceived, drafted and revised this work. I.V.E., A.C.G., A.G., K.S.O., R.S.S. and N.S. conceptualized and drafted the figures.
Corresponding authors
Ethics declarations
Competing interests
D.W.D. and family hold founder shares in F2G, a University of Manchester spin-out antifungal discovery company, and share options in TFF Pharma. D.W.D. acts or has recently acted as a consultant to Pulmatrix, Pulmocide, Biosergen, TFF Pharmaceuticals, Pfizer, Omega, Novacyt, Rostra Therapeutics, MucPharm, Mundipharma, Lifemine and Cipla; chairs a Data Review Committee for Pulmocide; and acts as a Phase 1 Medical Monitor for Biosergen. In the past three years, D.W.D. has been paid for talks on behalf of BioRad, Basilea and Pfizer. J.E.S. is a paid consultant for Zymergen, Sincarne and Michroma. L.E.C. is a co-founder of and shareholder in Bright Angel Therapeutics, a platform company for the development of novel antifungal therapeutics, and a Science Advisor for Kapoose Creek, a company that harnesses the therapeutic potential of fungi.
Peer review
Peer review information
Nature thanks Bernhard Hube and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Case, N.T., Gurr, S.J., Fisher, M.C. et al. Fungal impacts on Earth’s ecosystems. Nature 638, 49–57 (2025). https://doi.org/10.1038/s41586-024-08419-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08419-4
This article is cited by
-
Indigenizing fungal biotechnology for planetary health: an opinion paper
Fungal Biology and Biotechnology (2025)