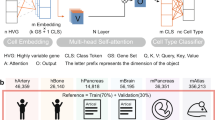
Abstract
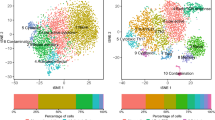
Tissue-level and organism-level biological processes often involve the coordinated action of multiple distinct cell types. The recent application of single-cell assays to many individuals should enable the study of how donor-level variation in one cell type is linked to that in other cell types. Here we introduce a computational approach called single-cell interpretable tensor decomposition (scITD) to identify common axes of interindividual variation by considering joint expression variation across multiple cell types. scITD combines expression matrices from each cell type into a higher-order matrix and factorizes the result using the Tucker tensor decomposition. Applying scITD to single-cell RNA-sequencing data on 115 persons with lupus and 83 persons with coronavirus disease 2019, we identify patterns of coordinated cellular activity linked to disease severity and specific phenotypes, such as lupus nephritis. scITD results also implicate specific signaling pathways likely mediating coordination between cell types. Overall, scITD offers a tool for understanding the covariation of cell states across individuals, which can yield insights into the complex processes that define and stratify disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The IFNβ stimulation dataset7 can be obtained from the Gene Expression Omnibus (GEO) under accession number GSE96583. The count matrices for the Perez et al. SLE scRNA-seq dataset9 can be obtained from the GEO under accession number GSE174188. The Nehar-Belaid et al. pSLE scRNA dataset11 can be obtained from the GEO under accession number GSE135779. The Stephenson et al. COVID-19 dataset is publicly available at https://www.covid19cellatlas.org/index.patient.html, titled ‘COVID-19 PBMC Ncl-Cambridge-UCL’. The van der Wijst et al. COVID-19 dataset can be found at https://cellxgene.cziscience.com/collections/7d7cabfd-1d1f-40af-96b7-26a0825a306d. The CellChat LR pair database can be found at https://github.com/LewisLabUCSD/Ligand-Receptor-Pairs/blob/master/Human/Human-2020-Jin-LR-pairs.csv. NicheNet regulatory potential scores were obtained from https://zenodo.org/record/3260758/files/ligand_target_matrix.rds. NicheNet ligand treatment datasets were obtained from https://zenodo.org/record/3260758/files/expression_settings.rds.
Code availability
Our computational method, scITD55, can be found on GitHub (https://github.com/kharchenkolab/scITD) or the Comprehensive R Archive Network (CRAN) (https://cloud.r-project.org/web/packages/scITD/index.html). A walkthrough tutorial for using scITD is also available at http://pklab.med.harvard.edu/jonathan/. The code used to produce all figures in this paper can be found at https://github.com/j-mitchel/scITD-Analysis/tree/main/figure_generation.
References
Kang, H. M. et al. Multiplexed droplet single-cell RNA-sequencing using natural genetic variation. Nat. Biotechnol. 36, 89–94 (2018).
Stoeckius, M. et al. Cell hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 19, 224 (2018).
Jerby-Arnon, L. & Regev, A. DIALOGUE maps multicellular programs in tissue from single-cell or spatial transcriptomics data. Nat. Biotechnol. 40, 1467–1477 (2022).
Ramirez Flores, R. O., Lanzer, J. D., Dimitrov, D., Velten, B. & Saez-Rodriguez, J. Multicellular factor analysis of single-cell data for a tissue-centric understanding of disease. eLife 12, e93161 (2023).
Tucker, L. R. Some mathematical notes on three-mode factor analysis. Psychometrika 31, 279–311 (1966).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 9, 559 (2008).
Targ, S. Multiplexing droplet-based single cell RNA-sequencing using genetic barcodes. Gene Expression Omnibus https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE96583 (2017).
Perez, R. K. et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science 376, eabf1970 (2022).
Perez, R. K. et al. Multiplexed scRNA-seq reveals the cellular and genetic correlates of systemic lupus erythematosus. Gene Expression Omnibus https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE174188 (2021).
Nehar-Belaid, D. et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 21, 1094–1106 (2020).
Nehar-Belaid, D., Flynn, W. F., Banchereau, J., Pascual, V. & Robson, P. A single cell approach to map cellular subsets involved in systemic lupus erythematosus (SLE) heterogeneity. Gene Expression Omnibus https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE135779 (2020).
Hooks, J. J. et al. Immune interferon in the circulation of patients with autoimmune disease. New Engl. J. Med. 301, 5–8 (1979).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Kirou, K. A. et al. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Nikpour, M., Dempsey, A. A., Urowitz, M. B., Gladman, D. D. & Barnes, D. A. Association of a gene expression profile from whole blood with disease activity in systemic lupus erythaematosus. Ann. Rheum. Dis. 67, 1069–1075 (2008).
Weckerle, C. E. et al. Network analysis of associations between serum interferon α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 63, 1044–1053 (2011).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Crow, M. K., Kirou, K. A. & Wohlgemuth, J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity 36, 481–490 (2003).
Feng, X. et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 54, 2951–2962 (2006).
Iwata, Y. et al. p38 mitogen-activated protein kinase contributes to autoimmune renal injury in MRL-Faslpr mice. J. Am. Soc. Nephrol. 14, 57–67 (2003).
Jin, N. et al. The selective p38 mitogen-activated protein kinase inhibitor, SB203580, improves renal disease in MRL/lpr mouse model of systemic lupus. Int. Immunopharmacol. 11, 1319–1326 (2011).
Azodi, C. B., Zappia, L., Oshlack, A. & McCarthy, D. J. splatPop: simulating population scale single-cell RNA sequencing data. Genome Biol. 22, 341 (2021).
Klumpe, H. et al. The context-dependent, combinatorial logic of BMP signaling. Cell Syst. 13, 388–407 (2022).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Dodeller, F. & Schulze-Koops, H. The p38 mitogen-activated protein kinase signaling cascade in CD4 T cells. Arthritis Res. Ther. 8, 205 (2006).
Wikenheiser, D. J. & Stumhofer, J. S. ICOS co-stimulation: friend or foe?. Front. Immunol. 7, 304 (2016).
Katan, M. B. Apolipoprotein E isoforms, serum cholesterol and cancer. Lancet 1, 507–508 (1986).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Burgess, S., Small, D. S. & Thompson, S. G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 26, 2333–2355 (2017).
Fox, J., Kleiber, C., Zeileis, A. & Kuschnig, N. ivreg: instrumental-variables regression by ‘2SLS’, ‘2SM’, or ‘2SMM’, with diagnostics. The Comprehensive R Archive Network https://zeileis.github.io/ivreg/ (2023).
Chen, L. et al. Genetic drivers of epigenetic and transcriptional variation in human immune cells. Cell 167, 1398–1414 (2016).
Quach, H. et al. Genetic adaptation and neandertal admixture shaped the immune system of human populations. Cell 167, 643–656 (2016).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53, 1300–1310 (2021).
Chun, S. et al. Limited statistical evidence for shared genetic effects of eQTLs and autoimmune disease-associated loci in three major immune cell types. Nat. Genet. 49, 600–605 (2017).
Stephenson, E. et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat. Med. 27, 904–916 (2021).
Cruikshank, W. W., Berman, J. S., Theodore, A. C., Bernardo, J. & Center, D. M. Lymphokine activation of T4+ T lymphocytes and monocytes. J. Immunol. 138, 3817–3823 (1987).
Winkler, M. S. et al. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: a diagnostic tool for immunosuppression? PLoS ONE 12, e0182427 (2017).
Olwal, C. O. et al. Parallels in sepsis and COVID-19 conditions: implications for managing severe COVID-19. Front. Immunol. 12, 91 (2021).
Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000 (2020).
Spinetti, T. et al. Reduced monocytic human leukocyte antigen-DR expression indicates immunosuppression in critically ill COVID-19 patients. Anesth. Analg. 131, 993–999 (2020).
van der Wijst, M. G. P. et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 13, eabh2624 (2021).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Barkas, N., Petukhov, V., Kharchenko, P. V. & Biederstedt, E. pagoda2: single cell analysis and differential expression. The Comprehensive R Archive Network https://github.com/kharchenkolab/pagoda2 (2021).
Li, J., Bien, J. & Wells, M. T. rTensor: an R package for multidimensional array (tensor) unfolding, multiplication, and decomposition. J. Stat. Softw. 87, 1–31 (2018).
Sheehan, B. N. & Saad, Y. Higher order orthogonal iteration of tensors (HOOI) and its relation to PCA and GLRAM. In Proceedings of the 2007 SIAM International Conference on Data Mining (eds Apte, C., Liu, B., Parthasarathy, S. & Skillicorn, D) (Society for Industrial and Applied Mathematics, 2007).
Kolda, T. G. & Bader, B. W. Tensor decompositions and applications. SIAM Rev. 51, 455–500 (2009).
Unkel, S., Hannachi, A., Trendafilov, N. T. & Jolliffe, I. T. Independent component analysis for three-way data with an application from atmospheric. J. Agric. Biol. Environ. Stat. 16, 319–338 (2011).
Zhou, G. & Cichocki, A. Fast and unique tucker decompositions via multiway blind source separation. Bull. Pol. Acad. Sci. Tech. Sci. 60, 389–405 (2012).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Johnson, W. E., Li, C. & Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2007).
Badea, L. Extracting gene expression profiles common to colon and pancreatic adenocarcinoma using simultaneous nonnegative matrix factorization. Pac. Symp. Biocomput. 2008, 267–278 (2008).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Shabalin, A. A. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 28, 1353–1358 (2012).
Wu, Y. et al. Integrative analysis of omics summary data reveals putative mechanisms underlying complex traits. Nat. Commun. 9, 918 (2018).
Mitchel, J., Biederstedt, E. & Kharchenko, P. V. Single-cell analysis of inter-individual variability by interpretable tensor decomposition. GitHub https://github.com/kharchenkolab/scITD (2024).
Acknowledgements
We thank S. Sunyaev (Harvard Medical School) for advice with the eQTL colocalization analysis and A. Igolkina (Gregor Mendel Institute) for advice on the cell proportion analysis.
Author information
Authors and Affiliations
Contributions
P.V.K. formulated the study and, with J.M., developed the overall approach. J.M. developed the detailed algorithms with advice from P.V.K. and assistance from E.B. J.M., C.J.Y. and P.V.K. worked on the interpretation of results, with help from M.G.G., R.K.P. and R.B. J.M. and P.V.K. drafted the paper, with contributions from C.J.Y. and input from the other authors.
Corresponding authors
Ethics declarations
Competing interests
P.V.K. is an employee of Altos Labs. C.J.Y. is a scientific advisory board member for and holds equity in Related Sciences and ImmunAI, is a consultant for and holds equity in Maze Therapeutics and is a consultant for Trex Bio. C.J.Y. has received research support from Chan Zuckerberg Initiative, Chan Zuckerberg Biohub and Genentech. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Notes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mitchel, J., Gordon, M.G., Perez, R.K. et al. Coordinated, multicellular patterns of transcriptional variation that stratify patient cohorts are revealed by tensor decomposition. Nat Biotechnol (2024). https://doi.org/10.1038/s41587-024-02411-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-024-02411-z
This article is cited by
-
Cross-tissue multicellular coordination and its rewiring in cancer
Nature (2025)
-
LaGrACE: estimating gene program dysregulation with latent regulatory network
Molecular Systems Biology (2025)