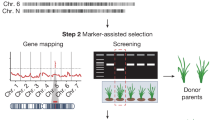
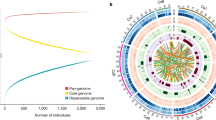
Abstract
Many thousands and, in some cases, millions of individuals from the major crop and livestock species have been genotyped and phenotyped for the purpose of genomic selection. ‘Ultimate genotypes’, in which the marker allele haplotypes with the most favorable effects on a target trait or traits in the population are combined together in silico, can be constructed from these datasets. Ultimate genotypes display up to six times the performance of the current best individuals in the population, as demonstrated for net profit in dairy cattle (incorporating a range of economic traits), yield in wheat and 100-seed weight in chickpea. However, current breeding strategies that aim to assemble ultimate genotypes through conventional crossing take many generations. As a hypothetical thought piece, here, we contemplate three future pathways for rapidly achieving ultimate genotypes: accelerated recombination with gene editing, direct editing of whole-genome haplotype sequences and synthetic biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Watson, I. A. & Singh, D. The future of rust resistant wheat in Australia. J. Aust. Inst. Agric. Sci. 28, 190–197 (1952).
Pedersen, W. L. & Leath, S. Pyramiding major genes for resistance to maintain residual effects. Annu. Rev. Phytopathol. 26, 369–378 (1988).
Peleman, J. D. & van der Voort, J. R. Breeding by design. Trends Plant Sci. 8, 330–334 (2003).
Hospital, F. Marker-assisted breeding. In Plant Molecular Breeding (ed. Newbury, H. J.) 30–56 (Blackwell Scientific, 2003).
Dekkers, J. C. M. & Hospital, F. The use of molecular genetics in the improvement of agricultural populations. Nat. Rev. Genet. 3, 22–32 (2002).
Servin, B., Martin, O. C., Mézard, M. & Hospital, F. Toward a theory of marker-assisted gene pyramiding. Genetics 168, 513–523 (2004).
Kemper, K. E., Bowman, P. J., Pryce, J. E., Hayes, B. J. & Goddard, M. E. Long-term selection strategies for complex traits using high-density genetic markers. J. Dairy Sci. 95, 4646–4656 (2012).
Cole, J. B. & VanRaden, P. M. Use of haplotypes to estimate Mendelian sampling effects and selection limits. J. Anim. Breed. Genet. 128, 446–455 (2011).
Georges, M., Charlier, C. & Hayes, B. Harnessing genomic information for livestock improvement. Nat. Rev. Genet. 20, 135–156 (2019).
Goddard, M. E. A mixed model for analyses of data on multiple genetic markers. Theor. Appl. Genet. 83, 878–886 (1992).
Kemper, K. E., Saxton, S. J., Bolormaa, S., Hayes, B. J. & Goddard, M. E. Selection for complex traits leaves little or no classic signatures of selection. BMC Genomics 15, 246 (2014).
Voss-Fels, K. P. et al. Breeding improves wheat productivity under contrasting agrochemical input levels. Nat. Plants 5, 706–714 (2019).
Varshney, R. K. et al. A chickpea genetic variation map based on the sequencing of 3,366 genomes. Nature 599, 622–627 (2021).
Villiers, K. et al. Evolutionary computing to assemble standing genetic diversity and achieve long-term genetic gain. Plant Genome 30, e20467 (2024).
Watson, A. et al. Multivariate genomic selection and potential of rapid indirect selection with speed breeding in spring wheat. Crop Sci. 59, 1945–1959 (2019).
Dinglasan, E. Integrating AI and genomic selection to sustain long-term genetic gain. Proc. PAG Aust. 1, 5095 (2023).
Xu, P., Wang, L. & Beavis, W. D. An optimization approach to gene stacking. Eur. J. Oper. Res. 214, 168–178 (2011).
Goiffon, M., Kusmec, A., Wang, L., Hu, G. & Schnable, P. S. Improving response in genomic selection with a population-based selection strategy: optimal population value selection. Genetics 206, 1675–1682 (2017).
Han, Y., Cameron, J. N., Wang, L. & Beavis, W. D. The predicted cross value for genetic introgression of multiple alleles. Genetics 205, 1409–1423 (2017).
Goszczynski, D. E., Denicol, A. C. & Ross, P. J. Gametes from stem cells: status and applications in animal reproduction. Reprod. Domest. Anim. 54, 22–31 (2019).
Young, N. D. & Tanksley, S. D. RFLP analysis of the size of chromosomal segments retained around the Tm-2 locus of tomato during backcross breeding. Theor. Appl. Genet. 77, 353–359 (1989).
Chinnici, J. P. Modification of recombination frequency in Drosophila. I. Selection for increased and decreased crossing over. Genetics 69, 71–83 (1971).
Bauer, E. et al. Intraspecific variation of recombination rate in maize. Genome Biol. 14, R103 (2013).
Johnston, S. E., Slate, J. & Pemberton, J. M. A genomic region containing RNF212 is associated with sexually-dimorphic recombination rate variation in wild Soay sheep (Ovis aries). Genetics 203, 583–598 (2016).
Sandor, C. et al. Genetic variants in REC8, RNF212, and PRDM9 influence male recombination in cattle. PLoS Genet. 8, e1002854 (2012).
Filler-Hayut, S., Melamed Bessudo, C. & Levy, A. Targeted recombination between homologous chromosomes for precise breeding in tomato. Nat. Commun. 8, 15605 (2017).
Filler-Hayut, S., Kniazev, K., Melamed-Bessudo, C. & Levy, A. A. Targeted inter-homologs recombination in Arabidopsis euchromatin and heterochromatin. Int. J. Mol. Sci. 9, 12096 (2021).
Xie, D. et al. Efficient targeted recombination with CRISPR/Cas9 in hybrids of Caenorhabditis nematodes with suppressed recombination. BMC Biol. 21, 203 (2023).
Fayos, I. et al. Manipulation of meiotic recombination to hasten crop improvement. Biology 11, 369 (2022).
Battagin, M. et al. Effect of manipulating recombination rates on response to selection in livestock breeding programs. Genet. Sel. Evol. 48, 44 (2016).
Taagen, E., Jordan, K., Akhunov, E., Sorrells, M. E. & Jannink, J. L. If it ain’t broke, don’t fix it: evaluating the effect of increased recombination on response to selection for wheat breeding. G3 12, jkac291 (2022).
Luo, J. et al. Pyramiding favorable alleles in an elite wheat variety in one generation by CRISPR–Cas9-mediated multiplex gene editing. Mol. Plant 14, 847–850 (2021).
Tanihara, F. et al. One-step generation of multiple gene-edited pigs by electroporation of the CRISPR/Cas9 system into zygotes to reduce xenoantigen biosynthesis. Int. J. Mol. Sci. 22, 2249 (2021).
Zsögön, A. et al. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 36, 1211–1216 (2018).
Martin-Rufino, J. D. et al. Massively parallel base editing to map variant effects in human hematopoiesis. Cell 186, 2456–2474 (2023).
Stuttmann, J. et al. Highly efficient multiplex editing: one-shot generation of 8× Nicotiana benthamiana and 12× Arabidopsis mutants. Plant J. 106, 8–22 (2022).
Lorenzo, C. D. et al. BREEDIT: a multiplex genome editing strategy to improve complex quantitative traits in maize. Plant Cell 35, 218–238 (2023).
Goddard, M. E. & Hayes, B. J. Mapping genes for complex traits in domestic animals and their use in breeding programmes. Nat. Rev. Genet. 10, 381–391 (2009).
Hayes, B. J. & Daetwyler, H. D. 1000 Bull Genomes Project to map simple and complex genetic traits in cattle: applications and outcomes. Annu. Rev. Anim. Biosci. 7, 89–102 (2019).
Krusnauskas, R. & Liu, Q. Haplotype-based gene editing for the treatment of RP1-associated IRDs. Invest. Ophthalmol. Vis. Sci. 65, 1029 (2024).
Warburton, C. L. Genomic Selection in Multi-Breed and Multi-Subspecies Populations. PhD thesis, University of Queensland (2024).
Cole, J. B., Null, D. J. & VanRaden, P. M. Phenotypic and genetic effects of recessive haplotypes on yield, longevity, and fertility. J. Dairy Sci. 99, 7274–7288 (2016).
Pryce, J. E. et al. A validated genome-wide association study in 2 dairy cattle breeds for milk production and fertility traits using variable length haplotypes. J. Dairy Sci. 93, 3331–3345 (2010).
Anand, R. P. et al. Design and testing of a humanized porcine donor for xenotransplantation. Nature 622, 393–401 (2023).
Niu, D. et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR–Cas9. Science 357, 1303–1307 (2017).
Che, P. et al. Wuschel2 enables highly efficient CRISPR/Cas-targeted genome editing during rapid de novo shoot regeneration in sorghum. Commun. Biol. 5, 344 (2022).
Lowe, K. et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28, 1998–2015 (2016).
Ellison, E. E. et al. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants 6, 620–624 (2020).
Massel, K. et al. Endogenous U6 promoters improve CRISPR/Cas9 editing efficiencies in Sorghum bicolor and show potential for applications in other cereals. Plant Cell Rep. 41, 489–492 (2022).
Zhao, Y. et al. Debugging and consolidating multiple synthetic chromosomes reveals combinatorial genetic interactions. Cell 186, 5220–5236 (2023).
Chen, L. G. et al. A designer synthetic chromosome fragment functions in moss. Nat. Plants 10, 228–239 (2024).
Coradini, A. L. V. et al. Building synthetic chromosomes from natural DNA. Nat. Commun. 14, 8337 (2023).
Bajpai, B. High capacity vectors. In Advances in Biotechnology (eds Ravi, I. et al.) (Springer, 2014).
Brown, D. M. et al. Efficient size-independent chromosome delivery from yeast to cultured cell lines. Nucleic Acids Res. 45, e50 (2017).
Pandey, S. et al. Efficient site-specific integration of large genes in mammalian cells via continuously evolved recombinases and prime editing. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-024-01227-1 (2024).
Kazuki, Y. & Oshimura, M. Human artificial chromosomes for gene delivery and the development of animal models. Mol. Ther. 19, 1591–1601 (2011).
Wang, M. L., Lin, X. J., Mo, B. X. & Kong, W. W. Plant artificial chromosomes: construction and transformation. ACS Synth. Biol. 13, 15–24 (2024).
Kuroiwa, Y. et al. Antigen-specific human polyclonal antibodies from hyperimmunized cattle. Nat. Biotechnol. 27, 173–181 (2009).
Martella, A., Pollard, S. M., Dai, J. & Cai, Y. Mammalian synthetic biology: time for big MACs. ACS Synth. Biol. 5, 1040–1049 (2016).
Gambogi, C. W. et al. Efficient formation of single-copy human artificial chromosomes. Science 383, 1344–1349 (2024).
Laurie, C. C. et al. The genetic architecture of response to long-term artificial selection for oil concentration in the maize kernel. Genetics 168, 2141–2155 (2004).
Dunnington, E. A. & Siegel, P. B. Long-term selection for 8-week body weight in chickens — direct and correlated responses. Theor. Appl. Genet. 71, 305–313 (1985).
Brotherstone, S. & Goddard, M. E. Artificial selection and maintenance of genetic variance in the global dairy cow population. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 1479–1488 (2005).
Yengo, L. et al. A saturated map of common genetic variants associated with human height. Nature 610, 704–712 (2022).
Hayes, B. J. et al. Accuracy of marker-assisted selection with single markers and marker haplotypes in cattle. Genet. Res. 89, 215–220 (2007).
Guinan, F. L. et al. Changes in genetic trends in US dairy cattle since the implementation of genomic selection. J. Dairy Sci. 106, 1110–1129 (2023).
Hill, W. G., Goddard, M. E. & Visscher, P. M. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 4, e1000008 (2008).
Yadav, S. et al. Improved genomic prediction of clonal performance in sugarcane by exploiting non-additive genetic effects. Theor. Appl. Genet. 134, 2235–2252 (2021).
Carlborg, Ö. et al. Epistasis and the release of genetic variation during long-term selection. Nat. Genet. 38, 418–420 (2006).
Barton, N. H. & Keightley, P. D. Understanding quantitative genetic variation. Nat. Rev. Genet. 3, 11–21 (2002).
Mulder, H. A., Lee, S. H., Clark, S., Hayes, B. J. & van der Werf, J. H. The impact of genomic and traditional selection on the contribution of mutational variance to long-term selection response and genetic variance. Genetics 213, 361–378 (2019).
Cooper, M. et al. Tackling G × E × M interactions to close on-farm yield-gaps: creating novel pathways for crop improvement by predicting contributions of genetics and management to crop productivity. Theor. Appl. Genet. 134, 1625–1644 (2021).
Scott, B. A., Haile-Mariam, M., Cocks, B. G. & Pryce, J. E. How genomic selection has increased rates of genetic gain and inbreeding in the Australian national herd, genomic information nucleus, and bulls. J. Dairy Sci. 104, 11832–11849 (2021).
Makanjuola, B. O. et al. Effect of genomic selection on rate of inbreeding and coancestry and effective population size of Holstein and Jersey cattle populations. J. Dairy Sci. 103, 5183–5199 (2020).
Tenhunen, S., Thomasen, J. R., Sørensen, L. P., Berg, P. & Kargo, M. Genomic analysis of inbreeding and coancestry in Nordic Jersey and Holstein dairy cattle populations. J. Dairy Sci. 107, 5897–5912 (2024).
Chari, R. & Church, G. Beyond editing to writing large genomes. Nat. Rev. Genet. 18, 749–760 (2017).
Sved, J. A. Linkage disequilibrium and homozygosity of chromosome segments in finite population. Theor. Pop. Biol. 2, 125–141 (1971).
Acknowledgements
We acknowledge M. Goddard and M. Morell for useful discussions. We thank the ARC Training Centre in Predictive Breeding for Agricultural for funding (IC230100016).
Author information
Authors and Affiliations
Contributions
B.J.H., I.D.G., T.J.M., K.V.-F., K.E.K. and L.T.H. conceived the main ideas presented in this Perspective and drafted the manuscript. K.V., C.W., E.D., H.R. and O.P. contributed to sections of the manuscript and helped draft the overall content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Alison Van Eenennaam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayes, B.J., Mahony, T.J., Villiers, K. et al. Potential approaches to create ultimate genotypes in crops and livestock. Nat Genet 56, 2310–2317 (2024). https://doi.org/10.1038/s41588-024-01942-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-024-01942-0
This article is cited by
-
Leveraging a phased pangenome for haplotype design of hybrid potato
Nature (2025)
-
A large-scale multi-environment study dissecting adult-plant resistance haplotypes for stripe rust resistance in Australian wheat breeding populations
Theoretical and Applied Genetics (2025)
-
The Farm Animal Genotype–Tissue Expression (FarmGTEx) Project
Nature Genetics (2025)