Abstract
Mitochondria contain a 16-kb double stranded DNA genome encoding 13 proteins essential for respiration, but the mechanisms regulating transcription and their potential role in cancer remain elusive. Although methyl-CpG-binding ___domain (MBD) proteins are essential for nuclear transcription, their role in mitochondrial DNA (mtDNA) transcription is unknown. Here we report that the MBD2c splicing variant translocates into mitochondria to mediate mtDNA transcription and increase mitochondrial respiration in triple-negative breast cancer (TNBC) cells. In particular, MBD2c binds the noncoding region in mtDNA and interacts with SIRT3, which in turn deacetylates and activates TFAM, a primary mitochondrial transcription factor, leading to enhanced mtDNA transcription. Furthermore, MBD2c recovered the decreased mitochondrial gene expression caused by the DNA synthesis inhibitor cisplatin, preserving mitochondrial respiration and consequently enhancing drug resistance and proliferation in TNBC cells. These data collectively demonstrate that MBD2c positively regulates mtDNA transcription, thus connecting epigenetic regulation by deacetylation with cancer cell metabolism, suggesting druggable targets to overcome resistance.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The ChIP−seq and BS-seq data have been deposited in the Gene Expression Omnibus (GEO) under the accession codes GSE263933 and GSE270688. The human genome sequence (hg19) downloaded from the UCSC genome browser (https://genome.ucsc.edu/) was used as the reference for mapping. Proteome Discovery version 1.3 using the MASCOT search engine was used to analyze protein acetylation data. All other data are available in Supplementary Information that are provided with this paper. Source data are provided with this paper.
References
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Li, F. et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol. 25, 6225–6234 (2005).
Asin-Cayuela, J. & Gustafsson, C. M. Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem. Sci. 32, 111–117 (2007).
Montoya, J., Christianson, T., Levens, D., Rabinowitz, M. & Attardi, G. Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proc. Natl Acad. Sci. USA 79, 7195–7199 (1982).
Bonekamp, N. A. & Larsson, N. G. SnapShot: mitochondrial nucleoid. Cell 172, 388–e381 (2018).
Shi, Y. H. et al. Mammalian transcription factor A is a core component of the mitochondrial transcription machinery. Proc. Natl Acad. Sci. USA 109, 16510–16515 (2012).
Kozhukhar, N. & Alexeyev, M. F. 35 years of TFAM research: old protein, new puzzles. Biology 12, 823 (2023).
Chatterjee, A. et al. MOF acetyl transferase regulates transcription and respiration in mitochondria. Cell 167, 722–738 (2016).
Arena, G. et al. Mitochondrial MDM2 regulates respiratory complex I activity independently of p53. Mol. Cell 69, 594–609 (2018).
Liu, Y. F. et al. Hypermethylation of mitochondrial DNA in vascular smooth muscle cells impairs cell contractility. Cell Death Dis. 11, 35 (2020).
Shu, Y. et al. Non-canonical phosphoglycerate dehydrogenase activity promotes liver cancer growth via mitochondrial translation and respiratory metabolism. EMBO J. 41, e111550 (2022).
Hallberg, B. M. & Larsson, N. G. Making proteins in the powerhouse. Cell Metab. 20, 226–240 (2014).
Lai, A. Y. & Wade, P. A. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat. Rev. Cancer 11, 588–596 (2011).
Lu, Y. et al. Alternative splicing of MBD2 supports self-renewal in human pluripotent stem cells. Cell Stem Cell 15, 92–101 (2014).
Gnanapragasam, M. N. et al. p66 ɑ−MBD2 coiled-coil interaction and recruitment of Mi-2 are critical for globin gene silencing by the MBD2−NuRD complex. Proc. Natl Acad. Sci. USA 108, 7487–7492 (2011).
Liu, Z. J. et al. Hypoxia-induced suppression of alternative splicing of MBD2 promotes breast cancer metastasis via activation of FZD1. Cancer Res. 81, 1265–1278 (2021).
Claros, M. G. & Vincens, P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 241, 779–786 (1996).
Santos, J. M. & Kowluru, R. A. Impaired transport of mitochondrial transcription factor A (TFAM) and the metabolic memory phenomenon associated with the progression of diabetic retinopathy. Diabetes Metab. Res. Rev. 29, 204–213 (2012).
Schwer, B., North, B. J., Frye, R. A., Ott, M. & Verdin, E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J. Cell Biol. 158, 647–657 (2002).
Marshall, C. & Parson, W. Interpreting NUMTs in forensic genetics: seeing the forest for the trees. Forensic Sci. Int. Genet. 53, 102497 (2021).
Du, Q., Luu, P. L., Stirzaker, C. & Clark, S. J. Methyl-CpG-binding ___domain proteins: readers of the epigenome. Epigenomics 7, 1051–1073 (2015).
Patil, V. et al. Human mitochondrial DNA is extensively methylated in a non-CpG context. Nucleic Acids Res. 47, 10072–10085 (2019).
Dou, X. Y. et al. The strand-biased mitochondrial DNA methylome and its regulation by DNMT3A. Genome Res. 29, 1622–1634 (2019).
Matsuda, S. et al. Accurate estimation of 5-methylcytosine in mammalian mitochondrial DNA. Sci. Rep. 8, 5801 (2018).
Guitton, R., Nido, G. S. & Tzoulis, C. No evidence of extensive non-CpG methylation in mtDNA. Nucleic Acids Res. 50, 9190–9194 (2022).
Yupeng He, J. R. E. Non-CG methylation in the human genome. Annu. Rev. Genomics Hum. Genet. 16, 55–77 (2015).
Campbell, C. T., Kolesar, J. E. & Kaufman, B. A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta Gene Regul. Mech. 1819, 921–929 (2012).
Kukat, C. et al. Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc. Natl Acad. Sci. USA 112, 11288–11293 (2015).
Gustafsson, C. M., Falkenberg, M. & Larsson, N. G. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 85, 133–160 (2016).
Freyer, C. et al. Maintenance of respiratory chain function in mouse hearts with severely impaired mtDNA transcription. Nucleic Acids Res. 38, 6577–6588 (2010).
King, G. A. et al. Acetylation and phosphorylation of human TFAM regulate TFAM−DNA interactions via contrasting mechanisms. Nucleic Acids Res. 46, 3633–3642 (2018).
Liu, H., Li, S. Y., Liu, X. H., Chen, Y. L. & Deng, H. T. SIRT3 overexpression inhibits growth of kidney tumor cells and enhances mitochondrial biogenesis. J. Proteome Res. 17, 3143–3152 (2018).
Akaki, K., Mino, T. & Takeuchi, O. DSP-crosslinking and immunoprecipitation to isolate weak protein complex. Bio Protoc. 12, e4478 (2022).
Li, S. T. et al. Myc-mediated SDHA acetylation triggers epigenetic regulation of gene expression and tumorigenesis. Nat. Metab. 2, 256–269 (2020).
Shu, X. T., Xiong, X. S., Song, J. H., He, C. & Yi, C. Q. Base-resolution analysis of cisplatin−DNA adducts at the genome scale. Angew. Chem. Int. Ed. 55, 14244–14247 (2016).
Cocetta, V., Ragazzi, E. & Montopoli, M. Mitochondrial involvement in cisplatin resistance. Int. J. Mol. Sci. 20, 3384 (2019).
Fan, X. L. et al. MiR-199a-3p enhances breast cancer cell sensitivity to cisplatin by downregulating TFAM (TFAM). Biomed. Pharmacother. 88, 507–514 (2017).
D’Souza, A. R. & Minczuk, M. Mitochondrial transcription and translation: overview. Essays Biochem. 62, 309–320 (2018).
Rubio-Cosials, A. et al. Human mitochondrial transcription factor A induces a U-turn structure in the light strand promoter. Nat. Struct. Mol. Biol. 18, 1281–1289 (2011).
Li, Y. F. & Li, Z. A. Potential mechanism underlying the role of mitochondria in breast cancer drug resistance and Its related treatment prospects. Front. Oncol. 11, 629614 (2021).
Obrist, F. et al. Metabolic vulnerability of cisplatin-resistant cancers. EMBO J. 37, e98597 (2018).
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E. & Gianni, L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13, 674–690 (2016).
Lynce, F. & Nunes, R. Role of platinums in triple-negative breast cancer. Curr. Oncol. Rep. 23, 50 (2021).
Sheth, S., Mukherjea, D., Rybak, L. P. & Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell Neurosci. 11, 338 (2017).
Caino, M. C. et al. A neuronal network of mitochondrial dynamics regulates metastasis. Nat. Commun. 7, 13730 (2016).
Lee, J. et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 568, 254–258 (2019).
Alam, T. I. et al. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 31, 1640–1645 (2003).
Tan, C. P. & Nakielny, S. Control of the DNA methylation system component MBD2 by protein arginine methylation. Mol. Cell. Biol. 26, 7224–7235 (2006).
Zhu, D. et al. BAI1 suppresses medulloblastoma formation by protecting p53 from Mdm2-mediated degradation. Cancer Cell 33, 1004–1016 (2018).
Mian, O. Y. et al. Methyl-binding ___domain protein 2-dependent proliferation and survival of breast cancer cells. Mol. Cancer Res. 9, 1152–1162 (2011).
Acknowledgements
This work is supported in part by the National Natural Science Foundation of China (82192893, 81930083 and 81821001 to H.Z., 82130087, 92357301 and 82341013 to P.G., 82422057 and 82273221 to L.S.), the Chinese Academy of Sciences (XDB0940101 to H.Z.), the National Key R&D Program of China (2022YFA1304504 to H.Z.), the Hefei Comprehensive National Science Center Institute of Health and Medicine Project (DJK-LX-2022001 to H.Z.), the Basic Funding of Guangzhou Municipal Science and Technology Bureau (2024A04J6493 to L.S.) and the Special Program for Young Scholars of Southern Medical University (G623281133 to L.S.).
Author information
Authors and Affiliations
Contributions
P.G. and H.Z. conceived the study and supervised experiments. Y.H., Z.Z., L.S., P.G. and H.Z. designed experiments. Y.H., Z.Z., R.L., H.L., Y.Z., Y.S., Q.M., T.Z., S.-T.L., Z.L. and Y.C. performed experiments. Y.H. and S.S. analyzed ChIP−seq data. Y.H., L.S., P.G. and H.Z. wrote the paper. All authors read and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Gordon Ginder and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
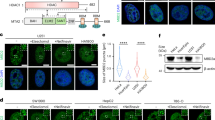
Extended Data Fig. 1 MBD2c trans-localizes into mitochondria via TOM20 and binds to the noncoding region.
a, WB analysis of the subcellular localization of endogenous MBD2c in MCF-10A, HBL-100, PLC, Huh7, THLE-3 and HEK-293T cells. Protein levels were assessed in whole-cell lysate (WCL), mitochondrial (Mito.), cytosolic (Cyto.), and nuclear (Nucl.) fractions, verified by Lamin B, Tubulin, and TOM20 protein levels as WB loading controls. b, WB analysis of the subcellular localization of Flag-tagged MBD2c in MDA-MB-468 and MDA-MB-231 cells. c, Schematic of MBD2c protein structure and selected deletion variants. Subcellular localization of Flag-MBD2c and indicated deletion variants in MDA-MB-468 cells was assessed by WB using anti-Flag antibodies in WCL and purified mitochondrial fraction, validated by Tubulin, Lamin B, and TOM20 protein levels. d, WB analysis of subcellular localization of endogenous MBD2c in MDA-MB-468 cells stably expressing NTC or shTOM20. VDAC was used as the loading control. e, HEK-293T cells were co-transfected with HA-TOM20 and Flag-MBD2c-WT or Flag-MBD2c-ΔC plasmids. Co-IP experiment was conducted using IgG or anti-Flag antibodies, followed by WB analysis. f, ChIP experiments were performed in MDA-MB-468 cells stably overexpressing Flag-MBD2c using IgG or anti-Flag antibodies. The noncoding region (NCR) occupancy by MBD2c was determined by qPCR, with atp8 and mt-co1 as the negative controls (n = 3 samples). g, ChIP-seq experiments conducted in MDA-MB-468 cells stably overexpressing Flag-tagged MTS-MBD2c, further infected with shTFAM-expressing lentivirus by using IgG or anti-Flag antibodies. Integrative Genomics Viewer (IGV) representation of ChIP-seq of MBD2c or BS-seq of 5mC displaying distribution encompassing the entire mitochondrial genome. The red line indicates the sequence of 5mC-modified HSP probes used in Fig. 1i. h, MedIP or ChIP&MedIP assays were conducted in MDA-MB-468 cells stably overexpressing Flag-tagged MTS-MBD2c. MedIP was carried out with IgG or anti-5mC antibodies. 5mC occupancy on NCR was assessed by qPCR (n = 3 samples). Immunoblots are representative of three independent experiments (a-e). In immunoprecipitations, approximately 10% of the supernatant was collected as input and equal volume of input and elution were loaded for WB. Error bars denote the mean ± S.E.M. (f,h). Statistical analyses were performed by two-tailed Student’s t-test (f,h).
Extended Data Fig. 2 MBD2c interacts with TFAM to promote mitochondrial transcription and respiration.
a-b, mtDNA-encoded mRNA levels and mtDNA contents were determined by qPCR in MDA-MB-468 cells with MBD2c KD (n = 3 samples). c, WB analysis of TFAM, TFB2M and POLRMT in MDA-MB-468 cells expressing NTC or shMBD2c. Actin served as the loading control. d, WB analysis of mtDNA-encoded protein levels in MDA-MB-231 and T-47D cells stably expressing NTC or shMBD2c. Actin served as the loading control. e, MDA-MB-468 cells with stable knockout of endogenous MBD2c, were further infected with viruses expressing EV, Flag-tagged MBD2c-WT, MTS-MBD2c or MBD2c-ΔC. WB analysis of subcellular localization of endogenous MBD2c and mtDNA-encoded proteins expression. Actin and TOM20 served as loading controls of whole-cell lysates or mitochondrial proteins, respectively. f, MDA-MB-468 cells expressing NTC or shMBD2c were further infected EV or Flag-tagged MTS-MBD2c lentivirus, followed by WB analysis to detect mtDNA-encoded proteins. Actin served as the loading control. g, WB analysis of mtDNA-encoded proteins levels in MDA-MB-468 cells stably expressing NTC or shTOM20. Actin served as the loading control. h, Co-IP assay was performed in HEK-293T cells transfected with Flag-TFAM and HA-MBD2c plasmids using anti-Flag antibodies. i, ChIP experiments were performed in MDA-MB-231 cells stably overexpressing Flag-TFAM, further infected with NTC or shMBD2c lentivirus by using IgG or anti-Flag antibodies. The NCR occupancy by TFAM was determined by qPCR (n = 3 samples). j, WB analysis of MBD2c and TFAM protein levels in MDA-MB-468 or MDA-MB-231 cells overexpressing Flag-TFAM that further infected with NTC or shMBD2c lentivirus. k, WB analysis of mtDNA-encoded protein levels in MDA-MB-468 cells stably expressing NTC or shTFAM. Actin served as the loading control. l-m, Cellular ATP concentrations and mitochondrial membrane potential (MMP) were detected in MDA-MB-468-shMBD2c cells further infected with EV or Flag-TFAM lentivirus (n = 3 samples). Immunoblots are representative of three independent experiments (c-h,j,k). In immunoprecipitations, approximately 10% of the supernatant was collected as input and equal volume of input and elution were loaded for WB. Error bars denote the mean ± S.E.M. (a,b,i,l,m). Statistical analyses were performed by two-tailed Student’s t-test (a,b,i,l,m).
Extended Data Fig. 3 MBD2c recruits SIRT3 to deacetylate and activate TFAM.
a, IP assay was performed using anti-TFAM antibodies in isolated mitochondria fractions from MDA-MB-468 cells treated with 10 mM NAM for 24 h, followed by WB analysis. b, IP assay using anti-MBD2c antibodies, was performed in MDA-MB-468 cells pre-treated by DSP, with or without KCC-07, followed by WB analysis. c, IP assay was performed in isolated mitochondria fractions using anti-MBD2c antibodies, with or without DNase I, followed by WB analysis. d, WB analysis of the levels of mtDNA-encoded proteins in MDA-MB-468 cells stably expressing NTC or shSIRT3. e, ChIP-qPCR was performed in MDA-MB-468 Flag-TFAM cells treated with KCC-07 or 3-TYP by using IgG or anti-Flag antibodies. The NCR occupancy by TFAM was determined by qPCR (n = 3 samples). f, IP was performed with anti-IgG or anti-Flag antibodies in cells mentioned in (e), followed by WB analysis. g, The diagram illustrates potential acetylated sites and structural ___domain of TFAM (upper panel). ChIP experiments were performed in MDA-MB-468 cells overexpressing Flag-tagged TFAMWT, TFAMK51&95&111Q, TFAMK145&146&154Q, and TFAMK181Q by using IgG or anti-Flag antibodies. The NCR occupancy was determined by qPCR (lower panel, n = 3 samples). h, MDA-MB-468-shMBD2c cells with stable expressing Flag-tagged TFAMWT, TFAMK145Q and TFAMK146Q, were further infected with sgTFAM viruses followed by WB analysis of mtDNA-encoded protein levels. i, qPCR detection to determine the mtDNA content in the cells mentioned in (h) (n = 3 samples). j, MDA-MB-468 cells with stably expressing EV, Flag-tagged TFAMWT, TFAMDKR and TFAMDKQ, were further infected with viruses to knockout endogenous TFAM, followed by qPCR detection of mt-RNA levels (n = 3 samples). k, MDA-MB-468 cells stably overexpressing Flag-tagged TFAMWT, or TFAMDKQ were further infected with NTC, shMBD2 or shSIRT3 lentivirus. IP was conducted using anti-Flag antibodies, followed by WB analysis. l, OCR were detected in cells mentioned in (k). The OCR curve was analyzed for basal respiration, maximal respiration, and ATP-linked respiration (n = 3 samples). Immunoblots are representative of three independent experiments (a-d,f,h,j). Error bars denote the mean ± S.E.M. (e,g,i,j,l). Statistical analyses were performed by two-tailed Student’s t-test (e,g,i,j,l).
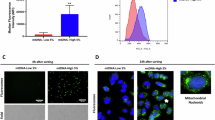
Extended Data Fig. 4 MBD2c-enhanced mitochondrial activity contributes to drug resistance in TNBC.
a, WB analysis of the proteins encoded by the nuclear genome in MDA-MB-468 cells treated with 4 μM or 10 μM CDDP for 72 or 96 h. b, Cell growth curves were analyzed by trypan blue counting in MDA-MB-468 cells treated with 4 μM CDDP and/or 5 μM GSH (n = 3 samples). c, Detection of OCR in MDA-MB-468 cells treated with 4 μM CDDP and/or 5 μM GSH for 72 h (n = 3 samples). d, Measurement of mitochondrial ROS levels using MitoSOX staining and flow cytometry in MDA-MB-468 cells mentioned in (b) (n = 3 samples). e, Calculation of IC50 curves for CDDP in MDA-MB-468 cells stably expressing EV or MTS-MBD2c (n = 3 samples). f, qPCR analysis of mtRNA levels in MDA-MB-468 cells expressing EV or MTS-MBD2c treated with 4 μM CDDP for 72 h (n = 3 samples). Immunoblots are representative of three independent experiments (a). Error bars denote the mean ± S.E.M. (b,c,e,f). Statistical analyses were performed by two-tailed Student’s t-test (f) or two-way ANOVA with Tukey’s multiple comparisons test (b).
Extended Data Fig. 5 TFAM acetylation enhanced breast cancer cell sensitivity to CDDP.
a, Measurement of cell death ratio in MDA-MB-468 cells expressing NTC or shMBD2c in the absence or presence of 4 μM CDDP (n = 3 samples). b, Plate colony formation assays were performed to measure the effect of CDDP (4 μM) on cell growth in the MDA-MB-468 cells expressing NTC or shMBD2c. c, Subcutaneous injection of MDA-MB-468 cells into nude mice. Intraperitoneal administration of 4 mg/kg CDDP began from day 10, and tumor growth curves were measured simultaneously (n = 5 mice in each group). d, WB analysis of mtDNA-encoded proteins in tumor tissues treated with or without 4 mg/kg CDDP. Actin served as the loading control. e, Tumor weights were calculated at the end of the experiment as shown in (Fig. 5g). f, WB analysis of TFAM, MBD2c, and mtDNA-encoded proteins in tumor tissues from each group as illustrated in (Fig. 5g). Actin served as the loading control. Plate colony formation assays (b) and immunoblots (d,f) are representative of three independent experiments. Error bars denote the mean ± S.E.M. (a,c,e). Statistical analyses were performed by two-tailed Student’s t-test (a,e) or two-way ANOVA with Tukey’s multiple comparisons test (c).
Extended Data Fig. 6 Gating strategies used in cell death analysis.
The gating strategy to identify the proportion of the cell death.
Supplementary information
Supplementary Information
Supplementary Tables 1–8.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hao, Y., Zhou, Z., Liu, R. et al. Mitochondria-localized MBD2c facilitates mtDNA transcription and drug resistance. Nat Chem Biol 21, 926–938 (2025). https://doi.org/10.1038/s41589-024-01776-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-024-01776-1
This article is cited by
-
The role of MBD2 in immune cell development, function, and autoimmune diseases
Cell Death Discovery (2025)