Abstract
Autoactivation of lineage-determining transcription factors mediates bistable expression, generating distinct cell phenotypes essential for complex body plans. Classical type 1 dendritic cell (cDC1) and type 2 dendritic cell (cDC2) subsets provide nonredundant functions for defense against distinct immune challenges. Interferon regulatory factor 8 (IRF8), the cDC1 lineage-determining transcription factor, undergoes autoactivation in cDC1 progenitors to establish cDC1 identity, yet its expression is downregulated during cDC2 differentiation by an unknown mechanism. This study reveals that the Irf8 +32-kb enhancer, responsible for IRF8 autoactivation, is naturally suboptimized with low-affinity IRF8 binding sites. Introducing multiple high-affinity IRF8 sites into the Irf8 +32-kb enhancer causes a gain-of-function effect, leading to erroneous IRF8 autoactivation in specified cDC2 progenitors, redirecting them toward cDC1 and a novel hybrid DC subset with mixed-lineage phenotypes. Further, this also causes a loss-of-function effect, reducing Irf8 expression in cDC1s. These developmental alterations critically impair both cDC1-dependent and cDC2-dependent arms of immunity. Collectively, our findings underscore the significance of enhancer suboptimization in the developmental segregation of cDCs required for normal immune function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The bulk RNA-seq data generated in this study on splenic DC populations are available in the Gene Expression Omnibus under accession number GSE241341. The scRNA-seq data generated in this study are available under accession number GSE270060. The following datasets were previously published and reanalyzed in this study: microarrays on DC progenitors and splenic DCs (GSE66565)22, and ChIP–seq for BATF3 and IRF8 in cDC1 (GSE66899)22. The following datasets were reanalyzed previously19 and used in the current study: ChIP–seq for p300, H3K4me1 and H3K27ac in DC progenitors and DCs (GSE66899)22, the assay for transposase-accessible chromatin with sequencing (ATAC-seq) of DC progenitors (GSE132240)23, and Immunological Genome Project (ImmGen) ATAC-seq on splenic DCs (GSE100738)68. Mouse genome (mm10 assembly) Bowtie 2 indexes were downloaded from the Bowtie 2 website (https://genome-idx.s3.amazonaws.com/bt/mm10.zip). Source data are provided with this paper. All other data are available in the paper or Supplementary Information.
References
Kuziora, M. A. & McGinnis, W. Autoregulation of a Drosophila homeotic selector gene. Cell 55, 477–485 (1988).
Thayer, M. J. et al. Positive autoregulation of the myogenic determination gene MyoD1. Cell 58, 241–248 (1989).
Ouyang, W. et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity 12, 27–37 (2000).
Liu, K. & Nussenzweig, M. C. Origin and development of dendritic cells. Immunol. Rev. 234, 45–54 (2010).
Murphy, T. L. et al. Transcriptional control of dendritic cell development. Annu. Rev. Immunol. 34, 93–119 (2016).
Anderson, D. A. III, Dutertre, C.-A., Ginhoux, F. & Murphy, K. M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 21, 101–115 (2021).
den Haan, J. M., Lehar, S. M. & Bevan, M. J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Durai, V. & Murphy, K. M. Functions of murine dendritic cells. Immunity 45, 719–736 (2016).
Lewis, K. L. et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity 35, 780–791 (2011).
Satpathy, A. T. et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat. Immunol. 14, 937–948 (2013).
Mayer, J. U. et al. Homeostatic IL-13 in healthy skin directs dendritic cell differentiation to promote TH2 and inhibit TH17 cell polarization. Nat. Immunol. 22, 1538–1550 (2021).
Kumamoto, Y. et al. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 39, 733–743 (2013).
Tussiwand, R. et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 42, 916–928 (2015).
Liu, T.-T. et al. Ablation of cDC2 development by triple mutations within the Zeb2 enhancer. Nature 607, 142–148 (2022).
Schiavoni, G. et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8α+ dendritic cells. J. Exp. Med. 196, 1415–1425 (2002).
Tussiwand, R. et al. Compensatory dendritic cell development mediated by BATF–IRF interactions. Nature 490, 502–507 (2012).
Glasmacher, E. et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1–IRF complexes. Science 338, 975–980 (2012).
Kim, S. et al. High amount of transcription factor IRF8 engages AP1–IRF composite elements in enhancers to direct type 1 conventional dendritic cell identity. Immunity 53, 759–774 (2020).
Onai, N. et al. Identification of clonogenic common Flt3+ M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 8, 1207–1216 (2007).
Naik, S. H. et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 8, 1217–1226 (2007).
Grajales-Reyes, G. E. et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α+ conventional DC clonogenic progenitor. Nat. Immunol. 16, 708–717 (2015).
Durai, V. et al. Cryptic activation of an Irf8 enhancer governs cDC1 fate specification. Nat. Immunol. 20, 1161–1173 (2019).
Farley, E. K. et al. Suboptimization of developmental enhancers. Science 350, 325–328 (2015).
Farley, E. K., Olson, K. M., Zhang, W., Rokhsar, D. S. & Levine, M. S. Syntax compensates for poor binding sites to encode tissue specificity of developmental enhancers. Proc. Natl Acad. Sci. USA 113, 6508–6513 (2016).
Jindal, G. A. & Farley, E. K. Enhancer grammar in development, evolution, and disease: dependencies and interplay. Dev. Cell 56, 575–587 (2021).
Hentsch, B., Mouzaki, A., Pfeuffer, I., Rungger, D. & Serfling, E. The weak, fine-tuned binding of ubiquitous transcription factors to the Il-2 enhancer contributes to its T cell-restricted activity. Nucleic Acids Res. 20, 2657–2665 (1992).
Jiang, J. & Levine, M. Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the dorsal gradient morphogen. Cell 72, 741–752 (1993).
Gaudet, J. & Mango, S. E. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 295, 821–825 (2002).
Scardigli, R., Bäumer, N., Gruss, P., Guillemot, F. & Le Roux, I. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development 130, 3269–3281 (2003).
Rowan, S. et al. Precise temporal control of the eye regulatory gene Pax6 via enhancer-binding site affinity. Genes Dev. 24, 980–985 (2010).
Swanson, C. I., Schwimmer, D. B. & Barolo, S. Rapid evolutionary rewiring of a structurally constrained eye enhancer. Curr. Biol. 21, 1186–1196 (2011).
Peterson, K. A. et al. Neural-specific Sox2 input and differential Gli-binding affinity provide context and positional information in Shh-directed neural patterning. Genes Dev. 26, 2802–2816 (2012).
Crocker, J. et al. Low affinity binding site clusters confer hox specificity and regulatory robustness. Cell 160, 191–203 (2015).
Jindal, G. A. et al. Single-nucleotide variants within heart enhancers increase binding affinity and disrupt heart development. Dev. Cell 58, 2206–2216 (2023).
Lim, F. et al. Affinity-optimizing enhancer variants disrupt development. Nature 626, 151–159 (2024).
Li, P. et al. BATF–JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Iwata, A. et al. Quality of TCR signaling determined by differential affinities of enhancers for the composite BATF–IRF4 transcription factor complex. Nat. Immunol. 18, 563–572 (2017).
Pigni, M., Ashok, D., Stevanin, M. & Acha-Orbea, H. Establishment and characterization of a functionally competent type 2 conventional dendritic cell line. Front. Immunol. 9, 1912 (2018).
Vremec, D. et al. Maintaining dendritic cell viability in culture. Mol. Immunol. 63, 264–267 (2015).
Sulczewski, F. B. et al. Transitional dendritic cells are distinct from conventional DC2 precursors and mediate proinflammatory antiviral responses. Nat. Immunol. 24, 1265–1280 (2023).
Rodrigues, P. F. et al. pDC-like cells are pre-DC2 and require KLF4 to control homeostatic CD4 T cells. Sci. Immunol. 8, eadd4132 (2023).
Rodrigues, P. F. et al. Progenitors of distinct lineages shape the diversity of mature type 2 conventional dendritic cells. Immunity 57, 1567–1585 (2024).
Bagadia, P. et al. An Nfil3–Zeb2–Id2 pathway imposes Irf8 enhancer switching during cDC1 development. Nat. Immunol. 20, 1174–1185 (2019).
Kashiwada, M., Pham, N. L., Pewe, L. L., Harty, J. T. & Rothman, P. B. NFIL3/E4BP4 is a key transcription factor for CD8{alpha}+ dendritic cell development. Blood 117, 6193–6197 (2011).
Liu, T.-T. et al. Cis interactions in the Irf8 locus regulate stage-dependent enhancer activation. Genes Dev. 37, 291–302 (2023).
Liu, Z. et al. Dendritic cell type 3 arises from Ly6C+ monocyte-dendritic cell progenitors. Immunity 56, 1761–1777 (2023).
Ferris, S. T. et al. cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature 584, 624–629 (2020).
Ardouin, L. et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity 45, 305–318 (2016).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Lun, Y., Sawadogo, M. & Perry, M. Autoactivation of Xenopus MyoD transcription and its inhibition by USF. Cell Growth Differ. 8, 275–282 (1997).
Datta, R. R. et al. A feed-forward relay integrates the regulatory activities of Bicoid and Orthodenticle via sequential binding to suboptimal sites. Genes Dev. 32, 723–736 (2018).
Stewart-Ornstein, J., Nelson, C., DeRisi, J., Weissman, J. S. & El-Samad, H. Msn2 coordinates a stoichiometric gene expression program. Curr. Biol. 23, 2336–2345 (2013).
Driever, W., Thoma, G. & Nüsslein-Volhard, C. Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature 340, 363–367 (1989).
Parker, D. S., White, M. A., Ramos, A. I., Cohen, B. A. & Barolo, S. The cis-regulatory logic of Hedgehog gradient responses: key roles for gli binding affinity, competition, and cooperativity. Sci. Signal. 4, ra38 (2011).
Ramos, A. I. & Barolo, S. Low-affinity transcription factor binding sites shape morphogen responses and enhancer evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130018 (2013).
Swanson, C. I., Evans, N. C. & Barolo, S. Structural rules and complex regulatory circuitry constrain expression of a Notch- and EGFR-regulated eye enhancer. Dev. Cell 18, 359–370 (2010).
Fukaya, T., Lim, B. & Levine, M. Enhancer control of transcriptional bursting. Cell 166, 358–368 (2016).
Heist, T., Fukaya, T. & Levine, M. Large distances separate coregulated genes in living Drosophila embryos. Proc. Natl Acad. Sci. USA 116, 15062–15067 (2019).
Du, M. et al. Direct observation of a condensate effect on super-enhancer controlled gene bursting. Cell 187, 331–344.e17 (2024).
Grant, C. E., Bailey, T. L. & Noble, W. S. FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018 (2011).
Kamizono, S. et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J. Exp. Med. 206, 2977–2986 (2009).
Briseño, C. G. et al. Distinct transcriptional programs control cross-priming in classical and monocyte-derived dendritic cells. Cell Rep. 15, 2462–2474 (2016).
Ou, F. et al. Enhanced in vitro type 1 conventional dendritic cell generation via the recruitment of hematopoietic stem cells and early progenitors by Kit ligand. Eur. J. Immunol. 53, e2250201 (2023).
Theisen, D. J. et al. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 362, 694–699 (2018).
Zhang, Y., Parmigiani, G. & Johnson, W. E. ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom. Bioinform 2, lqaa078 (2020).
Camberis, M., Le Gros, G. & Urban, J., Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. 55, 19.12.1–19.12.27 (2003).
Heng, T. S. P. et al. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Acknowledgements
This work was supported by grants from the US National Institute of Health (NIH) to K.M.M. (R01AI150297, R01CA248919, R21AI164142, R01AI162643 and R21AI163421). S.T.F. is a Cancer Research Institute Irvington Fellow supported by the Cancer Research Institute. We thank J. M. White at the Department of Pathology and Immunology Transgenic Mouse Core at Washington University in St. Louis and the Genetic Editing and iPS Cell Center at Washington University in St. Louis for generating the mouse models. We thank the GTAC@MGI at Washington University School of Medicine for sequencing services. We are grateful to C. Fan and C. A. Miller at Washington University School of Medicine, and Y. Zhou at Stanford University School of Medicine for their valuable advice on genomic analysis and S. Hui at Washington University School of Medicine for help with structural modeling. We acknowledge the NIH Tetramer Core Facility (contract no. 75N93020D00005) for providing SIINFEKL-Kb tetramers. We thank E. K. Farley at the University of California, San Diego, D. L. Stern at HHMI’s Janelia Research Campus and E. V. Rothenberg at the California Institute of Technology for insights about enhancer affinity and syntax.
Author information
Authors and Affiliations
Contributions
F.O., T.L.M. and K.M.M. designed the study. F.O., T.-T.L., P.D., S.T.F., S.K., H.S., R.A.O., S.J. and J.C. performed experiments with advice from J.L.P., S.D. and M.S.D. F.O. and K.M.M. wrote the manuscript with advice from T.L.M. and T.-T.L.
Corresponding author
Ethics declarations
Competing interests
M.S.D. is a consultant or advisor for Inbios, Vir Biotechnology, Moderna, Merck, GlaxoSmithKline, IntegerBio and Akagera Medicines. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Moderna, Vir Biotechnology, IntegerBio and Emergent BioSolutions. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: S. Houston, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The Irf8 + 32 kb comprises four AICE motifs.
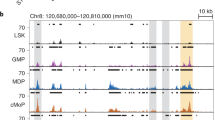
(a) ATAC-seq and ChIP-seq tracks for the indicated cell types are shown for the Irf8 locus. The locations of the Irf8 + 32 kb, +41 kb and +56 kb enhancers are highlighted. The tracks are displayed using the UCSC genome browser with vertical auto scale. (b) A zoomed-in view of a 1,168 bp region surrounding the Irf8 + 32 kb enhancer highlighting the locations of AICEs. The previously deleted 149 bp (blue) and 421 bp (red) regions from Irf8 + 32 5′−/− mice and Irf8 + 32−/− mice, respectively23, are indicated relative to the AICEs. (c) Sequence of the 421 bp region deleted previously in Irf8 + 32−/− mice23, with AICEs underlined and highlighted in blue. The AICEs are displayed from left to right in the order of site 1, site 4, site 3, and site 2.
Extended Data Fig. 2 AICEs in mouse and human IRF8 enhancers show low binding affinities to the BATF3-JUNB-IRF8 complexes.
(a) Competitive EMSA showing binding of 32P-dCTP-labeled Ctla4 probe with the indicated unlabeled competitors at increasing amounts (0, 25, 100, 400, and 1600−fold excess). The NEs used were combined from 293FT cells transfected with Junb and Batf3 and 293FT cells transfected with Irf8. Bands corresponding JUNB-BATF3-IRF8 complexes and free probes are indicated by the upper and lower arrows, respectively. Data shown are representative of two similar experiments. (b) EMSA performed with 32P-dCTP-labeled probes on various NEs: NEs from 293FT cells transfected with Junb and Batf3 (B), combined NEs from 293FT cells transfected with Junb and Batf3 and 293FT cells transfected with IRF8 (B + I), and NEs from MutuDCs. Bands corresponding JUNB-BATF3-IRF8 and JUNB-BATF3 complexes are indicated by the upper and lower arrows, respectively. Data shown are representative of three similar experiments. (c) Alignment of a 161 bp region encompassing the four AICEs in the mouse Irf8 + 32 kb enhancer (chr8:120,768,539-120,768,699, mm10) across several mammalian species, colored by conservation (Jalview). The six most probable AICEs (defined using FIMO and the AICE PWM from Fig. 1b) are indicated. (d) Retroviral reporter analysis of the mouse Irf8 + 32 kb enhancer and the human IRF8 + 48 kb enhancer in cDC1s and cDC2s obtained from KitL/Flt3L cultured mouse BM cells. Shown are enhancer-driven GFP expression in transduced cDC1s (pre-gate: B220- CD11c+ MHCII+ CD24+ Sirpa− Thy1.1+ cells) and transduced cDC2s (pre-gate: B220− CD11c+ MHCII+ Sirpa+ Thy1.1+ cells). Numbers are GFP geometric MFI. Data shown are representative of two similar experiments. (e) Competitive EMSA performed with 32P-dCTP-labeled Ctla4 probe in the presence of 200-fold excess of various unlabeled competitors. Competitors derived from the human +48 kb enhancer are denoted as ‘Hu’. Bands corresponding JUNB-BATF3-IRF8 complexes and free probes are indicated by the upper and lower arrows, respectively. Data shown are representative of two similar experiments.
Extended Data Fig. 3 Characterization of DC progenitors and splenic DC populations.
(a) Expression levels of selected TF-encoding genes in different stages of DC development assessed by microarray. (b) IRF8 Expression across different stages of DC development in WT mice as determined by intracellular staining. Numbers are geometric MFI. Data shown are representative of two similar experiments. MDP, monocyte-dendritic cell progenitors. (c) Frequencies of the indicated DC progenitors from WT, H/+, and H/H mice as percentages of lin− SiglecH− BM cells. Data are pooled from three independent experiments (n = 5 for WT, 3 for H/+, and 4 for H/H mice). Statistical significance was determined by one-way ANOVA. (d) Frequencies of splenic pDCs (gated as B220− SiglecH+ splenocytes) from WT, H/+, and H/H mice. Data are pooled from three independent experiments (n = 9 for WT, 5 for H/+, and 7 for H/H mice). Statistical significance was determined by one-way ANOVA. (e) ZBTB46 expression in the indicated cell types from H/H spleens measured by intracellular staining. B cells serve as a negative control for ZBTB46 expression. (f) In vitro cross-presentation assay with different DC populations. Shown are representative flow cytometry plots depicting CellTrace Violet-labeled OT-I CD8 T cells co-cultured with the indicated DC subsets and HKLM-OVA antigen for three days (pre-gate: CD45.2− CD45.1+ CD8a+ CD4− Va2+ cells). Divided OT-I cells indicate successful cross-presentation and T cell activation. (g) Frequencies of divided OT-I cells from in vitro cross-presentation assays. Data are pooled from two independent experiments. (h) Overlaid flow cytometry plots showing splenic cDC1s (cyan), cDC2s (red), and hybrid DCs (purple) before (top panel) and after (middle and bottom panels) culturing in the specified conditions. The data presented are representative of three similar experiments. Data in c and d are presented as mean values +/− SD.
Extended Data Fig. 4 An optimized Irf8 + 32 kb enhancer maintains IRF8 expression in pre-cDC2, impeding their lineage commitment to cDC2.
(a) IRF8 Expression in the indicated DC progenitors from WT and Irf8 + 32H/H (H/H) mice as determined by intracellular staining. Numbers are geometric MFI. Data shown are representative of two similar experiments. (b) Representative flow cytometry plots depicting intracellular IRF8 in WT and H/H pre-cDC2s after 18 hours of culturing in the presence of Flt3L. (c) Frequencies of IRF8hi pre-cDC2s after 18 h of culturing. Data are pooled from three independent experiments (n = 5 for WT and H/H mice). Statistical significance was determined by unpaired, two-tailed Student’s t test. (d) Representative flow cytometry plots showing splenic cDC populations from WT, Irf4−/−, H/H, and H/H Irf4−/− mice (pre-gate: B220− SiglecH− CD11c+ MHCII+ splenocytes). (e) Frequencies of splenic cDC2s and hybrid DCs from the indicated mice. Data are pooled from five independent experiments. Statistical significance was evaluated using one-way ANOVA with Dunnett’s multiple comparisons test. (f) Representative flow cytometry plot depicting cDCs differentiated from the indicated DC progenitors sorted from WT and H/H Irf4−/− BM and cultured in the presence of Flt3L for four days (pre-gate: B220− SiglecH− CD11c+ MHCII+ cells). (g) Frequencies of cDC1s differentiated from WT and H/H Irf4−/− pre-cDC2s. Data are pooled from three independent experiments. Statistical significance was determined by unpaired, two-tailed Student’s t test. Data in c, e, and g are presented as mean values +/− SD. P values are indicated above the graphs.
Extended Data Fig. 5 The Irf8 + 32H enhancer drives hybrid DC development independently of the Irf8 + 41 kb enhancer.
(a) Diagram illustrating the deletion of the Irf8 + 41 kb enhancer on the Irf8 + 32H/H background using CRISPR/Cas9. (b) Sequence of a 658 bp region surrounding the Irf8 + 41 kb enhancer. Nucleotides deleted in both the Irf8 + 41−/− line23 and the Irf8 + 32H/H + 41−/− line are highlighted in red. Additional nucleotides deleted only in the Irf8 + 32H/H + 41−/− line highlighted in blue. sgRNAs target sites are underlined. (c) Representative flow cytometry plots of BM cells stained for pre-cDC1 (upper panel), and splenocytes stained for cDCs (lower panel) from WT, Irf8 + 41−/− (+41−/−), Irf8 + 32H/+ + 41−/− (+32H/+ + 41−/−), and Irf8 + 32H/H + 41−/− (+32H/H + 41−/−) mice. Chromatin phasing diagrams are displayed above the plots for context. Pre-gates: lin− SiglecH− Flt3+ cells for BM, and B220− SiglecH− CD11c+ MHCII+ cells for spleen. (d) Frequencies of pre-cDC1s as a percentage of lin− SiglecH− BM cells across the indicated genotypes. Data are pooled from three independent experiments (n = 4 for WT, 5 for +41−/−, 5 for +32H/+ + 41−/−, and 4 for +32H/H + 41−/− mice). Statistical significance was determined by one-way ANOVA with Dunnett’s multiple comparison test. (e) Frequencies of splenic cDC1s, cDC2s and hybrid DCs across the indicated genotypes. Data are pooled from six independent experiments. Statistical significance was determined by one-way ANOVA with Dunnett’s multiple comparison test. (f) IRF8 expression in cDC2s and hybrid DCs from the indicated genotypes as determined by intracellular staining. Data shown are representative of two similar experiments. Data in d and e are presented as mean values +/− SD. P values are indicated above the graphs.
Extended Data Fig. 6 An optimized Irf8 + 32 kb enhancer drives convergent differentiation of pre-cDC1 and pre-cDC2 toward a hybrid DC phenotype.
(a) Left panel: heatmap visualization of the bulk RNA-seq data as in Fig. 4e. Shown are the top 464 genes differentially expressed between WT cDC1 and cDC2 identified in Fig. 2f. Right panel: zoomed-in views of selected clusters. (b) Schematics illustrating the differentiation of CDP into cDC1, cDC2, and hybrid DC in WT, H/+, and H/H mice. Cell types are color-coded based on IRF8 and BATF3 expression.
Extended Data Fig. 7 Analyses of intermediately optimized Irf8 + 32 kb enhancers.
(a) Diagrams and sequences of the different Irf8 + 32 kb enhancer alleles generated in this study. (b) Retroviral reporter activities of various Irf8 + 32 kb enhancer constructs in cDC1s obtained from Kit/Flt3L-cultured BM. Shown are enhancer-driven GFP expression in transduced cDC1s (pre-gate: B220− CD11c+ MHCII+ CD24+ Sirpa− Thy1.1+ cells). Numbers are GFP geometric MFI. Data shown are pooled from two independent experiments (n = 3 for WT mice). Statistical significance was determined by one-way ANOVA with Dunnett’s multiple comparison test. (c) Representative flow cytometry plots of splenocytes stained for cDCs (pre-gate: B220− SiglecH− CD11c+ MHCII+ splenocytes). (d) Frequencies of the indicated DC subsets as percentages of B220− SiglecH− CD11c+ MHCII+ cDCs. Data are pooled from six independent experiments. Statistical significance was evaluated using one-way ANOVA with Dunnett’s multiple comparisons test. (e) Representative flow cytometry plots of CD11c-enriched splenocytes stained for cDCs (pre-gate: B220− CD11c+ MHCII+ splenocytes). (f) Frequencies of the indicated DC subsets as percentages of B220− CD11c+ MHCII+ cDCs. Data are pooled from two independent experiments (n = 4 for WT and I/I mice). Statistical significance was determined by unpaired, two-tailed Student’s t test. (g) Representative histograms showing intracellular IRF8 levels in DC subsets gated in (e). Numbers are geometric MFI. (h) IRF8 geometric MFI in cDC1s gated in (e). Statistical significance was determined by unpaired, two-tailed Student’s t test. Data shown are pooled from two independent experiments (n = 4 for WT and I/I mice). Data in b, d, f, and h are presented as mean values +/− SD. P values are indicated above the graphs.
Extended Data Fig. 8 scRNA-seq analysis of WT and Irf8 + 32H/H BM and spleen.
(a) Clustering of BM lin− Flt3+ Kitint-lo cells from WT and H/H mice projected onto a UMAP space (22,839 total cells, with 11,441 from WT and 11,398 from H/H mice). (b) Distributions of WT (blue) and H/H (red) cells across the UMAP in (a). (c) Dot plot of top 10 differentially expressed genes for each cluster in (a). (d) Clustering of BM lin− Flt3+ Kitint-lo cells in (a) split by individual biological replicates. (e) Normalized and scaled Irf8 expression in WT (upper panel) and H/H (lower panel) cells. (f) Violin plot of normalized and scaled Irf8 expression across clusters. WT (blue) and H/H (red) cells are displayed side by side for each cluster. Statistical significance was determined by a two-tailed Wilcoxon rank sum test, applying a log2 fold-change threshold of 0.1. P values are indicated above the graphs for statistically significant comparisons. (g) Clustering of splenic CD11c+ cells from Fig. 5a split by individual biological replicates. (h) Normalized and scaled expressions of selected cluster marker genes in splenic CD11c+ cells.
Supplementary information
Supplementary Information
Supplementary Fig. 1: Gating strategies for flow cytometry.
Supplementary Tables 1–4
Supplementary Table 1: EMSA probes and competitors. Supplementary Table 2: Enhancer variants utilized in retroviral reporter assays. Supplementary Table 3: Antibodies used for flow cytometry. Supplementary Table 4: gRNAs, ssODN and genotyping primers related to the new mouse strains.
Source data
Source Data
Gel source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ou, F., Liu, TT., Desai, P. et al. Optimization of the Irf8 +32-kb enhancer disrupts dendritic cell lineage segregation. Nat Immunol 25, 2043–2056 (2024). https://doi.org/10.1038/s41590-024-01976-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-024-01976-w
This article is cited by
-
Evolving Rel
Nature Immunology (2025)