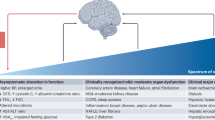
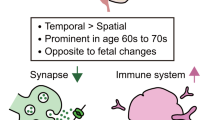
Abstract
Aging induces molecular, cellular and functional changes in the adult brain that drive cognitive decline and increase vulnerability to dementia-related neurodegenerative diseases. Leveraging systemic and lifestyle interventions, such as heterochronic parabiosis, administration of ‘young blood’, exercise and caloric restriction, has challenged prevalent views of brain aging as a rigid process and has demonstrated that aging-associated cognitive and cellular impairments can be restored to more youthful levels. Technological advances in proteomic and transcriptomic analyses have further facilitated investigations into the functional impact of intertissue communication on brain aging and have led to the identification of a growing number of pro-aging and pro-youthful factors in blood. In this review, we discuss blood-to-brain communication from a systems physiology perspective with an emphasis on blood-derived signals as potent drivers of both age-related brain dysfunction and brain rejuvenation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lehallier, B. et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 25, 1843–1850 (2019). This is an extensive resource of aging-associated changes of the human plasma proteome.
Schaum, N. et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Praag, H., van, Shubert, T., Zhao, C. & Gage, F. H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 (2005).
Kramer, A. F. et al. Ageing, fitness and neurocognitive function. Nature 400, 418–419 (1999).
Ingram, D. K., Weindruch, R., Spangler, E. L., Freeman, J. R. & Walford, R. L. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 42, 78–81 (1987).
Witte, A. V., Fobker, M., Gellner, R., Knecht, S. & Flöel, A. Caloric restriction improves memory in elderly humans. Proc. Natl Acad. Sci. USA 106, 1255–1260 (2009).
Horowitz, A. M. et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020). The beneficial effects of exercise during old age can be transferred through blood plasma and the liver-derived systemic enzyme GPLD1.
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011). This report demonstrates that systemic blood factors, including CCL11, impair cognitive and regenerative functions.
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014). This study provided evidence that blood plasma administration is sufficient to restore cognitive deficits in aged recipient mice.
Katsimpardi, L. et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 (2014). GDF11 treatment enhances vascular function and neurogenesis in the aged SVZ.
Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 (2016).
Fan, X., Wheatley, E. G. & Villeda, S. A. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu. Rev. Neurosci. 40, 251–272 (2017).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Wheatley, E. G. et al. Neuronal O-GlcNAcylation improves cognitive function in the aged mouse brain. Curr. Biol. 29, 3359–3369 (2019).
Mattson, M. P. & Arumugam, T. V. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 27, 1176–1199 (2018).
Simkin, D. et al. Aging-related hyperexcitability in CA3 pyramidal neurons is mediated by enhanced A-type K+ channel function and expression. J. Neurosci. 35, 13206–13218 (2015).
Gontier, G. et al. Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 22, 1974–1981 (2018).
Enwere, E. et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365 (2004).
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Negredo, P. N., Yeo, R. W. & Brunet, A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 27, 202–223 (2020).
Sorrells, S. F. et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381 (2018).
Boldrini, M. et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589–599 (2018).
Moreno-Jiménez, E. P. et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560 (2019).
Akers, K. G. et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014).
Sahay, A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011).
Wang, F. et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 23, 481–486 (2020).
Sierra, A., Gottfried‐Blackmore, A. C., McEwen, B. S. & Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424 (2007).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Stephan, A. H. et al. A dramatic increase of C1q protein in the CNS during normal aging. J. Neurosci. 33, 13460–13474 (2013).
Shi, Q. et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J. Neurosci. 35, 13029–13042 (2015).
Pluvinage, J. V. et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568, 187–192 (2019).
Clarke, L. E. et al. Normal aging induces A1-like astrocyte reactivity. Proc. Natl Acad. Sci. USA 115, E1896–E1905 (2018).
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N. & Allen, N. J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285 (2018).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Buckley, M. W. & McGavern, D. B. Immune dynamics in the CNS and its barriers during homeostasis and disease. Immunol. Rev. 306, 58–75 (2022).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Silva, T. M. D. & Faraci, F. M. Contributions of aging to cerebral small vessel disease. Annu. Rev. Physiol. 82, 275–295 (2019).
Ungvari, Z. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 15, 555–565 (2018).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019).
Soto, I. et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 13, e1002279 (2015).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25, 988–1000 (2019). This study characterizes aging BECs and the effect of VCAM1 on neuroinflammatory, regenerative and cognitive dysfunction with age.
Chen, M. B. et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 30, 4418–4432 (2020).
Yang, A. C. et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430 (2020). This study used a new and innovative approach to investigate BBB function and plasma protein transport with age.
Propson, N. E., Roy, E. R., Litvinchuk, A., Köhl, J. & Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood–brain barrier permeability during aging. J. Clin. Invest. 131, e140966 (2021).
Rebo, J. et al. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat. Commun. 7, 13363 (2016).
Yousef, H. et al. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget 6, 11959–11978 (2015).
Smith, L. K. et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 21, 932–937 (2015).
Smith, L. K. et al. The aged hematopoietic system promotes hippocampal‐dependent cognitive decline. Aging Cell 19, e13192 (2020).
Minhas, P. S. et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 590, 122–128 (2021). Targeting EP2 signaling in aged peripheral myeloid cells reduces systemic and brain inflammatory states and enhances synaptic plasticity and cognitive functions in aged animals.
Berry, K. et al. B and T lymphocyte densities remain stable with age in human cortex. ASN Neuro 13, 17590914211018116 (2021).
Jin, W.-N. et al. Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat. Neurosci. 24, 61–73 (2021).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019). This study identified a role for infiltrating immune cells on neuronal stem cell function in the aging SVZ.
Unger, M. S. et al. CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav. Immun. 89, 67–86 (2020).
Batterman, K. V., Cabrera, P. E., Moore, T. L. & Rosene, D. L. T cells actively infiltrate the white matter of the aging monkey brain in relation to increased microglial reactivity and cognitive decline. Front. Immunol. 12, 607691 (2021).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020).
Ransohoff, R. M. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31, 711–721 (2009).
Baruch, K. et al. CNS-specific immunity at the choroid plexus shifts toward destructive TH2 inflammation in brain aging. Proc. Natl Acad. Sci. USA 110, 2264–2269 (2013).
Das, M. M. et al. Young bone marrow transplantation preserves learning and memory in old mice. Commun. Biol. 2, 73 (2019). This study used heterochronic bone marrow transplantation to rejuvenate the aged brain.
Fernández-Castañeda, A. et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185, 2452–2468 (2022).
Erickson, M. A., Morofuji, Y., Owen, J. B. & Banks, W. A. Rapid transport of CCL11 across the blood–brain barrier: regional variation and importance of blood cells. J. Pharmacol. Exp. Ther. 349, 497–507 (2014).
Stamatovic, S. M. et al. Monocyte chemoattractant protein-1 regulation of blood–brain barrier permeability. J. Cereb. Blood Flow Metab. 25, 593–606 (2004).
Bettcher, B. M. et al. MCP-1 and eotaxin-1 selectively and negatively associate with memory in MCI and Alzheimer’s disease dementia phenotypes. Alzheimers Dement. 3, 91–97 (2016).
Lee, W.-J. et al. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Sci. Rep. 8, 1280 (2018).
Carrette, O. et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics 3, 1486–1494 (2003).
Brew, B. J., Dunbar, N., Pemberton, L. & Kaldor, J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J. Infect. Dis. 174, 294–298 (1996).
Starkey, H. D. V. et al. Neuroglial expression of the MHCI pathway and PirB receptor is upregulated in the hippocampus with advanced aging. J. Mol. Neurosci. 48, 111–126 (2012).
Huh, G. S. et al. Functional requirement for class I MHC in CNS development and plasticity. Science 290, 2155–2159 (2000).
Lin, K. et al. MHC class I H2-Kb negatively regulates neural progenitor cell proliferation by inhibiting FGFR signaling. PLoS Biol. 19, e3001311 (2021).
Bogeska, R. et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 29, 1273–1284 (2022).
Ho, T. T. et al. Aged hematopoietic stem cells are refractory to bloodborne systemic rejuvenation interventions. J. Exp. Med. 218, e20210223 (2021).
Jin, Z.-G. et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 1186–1191 (2004).
Tchalla, A. E. et al. Elevated soluble vascular cell adhesion molecule-1 is associated with cerebrovascular resistance and cognitive function. J. Gerontol. A Biol. Sci. Med. Sci. 72, 560–566 (2017).
Zuliani, G. et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J. Neurol. Sci. 272, 164–170 (2008).
Park, M. H. et al. Vascular and neurogenic rejuvenation in aging mice by modulation of ASM. Neuron 100, 167–182 (2018).
Biessels, G. J. & Despa, F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 14, 591–604 (2018).
Bocarsly, M. E. et al. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl Acad. Sci. USA 112, 15731–15736 (2015).
Cifre, M., Palou, A. & Oliver, P. Cognitive impairment in metabolically-obese, normal-weight rats: identification of early biomarkers in peripheral blood mononuclear cells. Mol. Neurodegener. 13, 14 (2018).
Parikh, I. et al. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging 8, 2814–2826 (2016).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Loffredo, F. S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Salpeter, S. J. et al. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes 62, 2843–2848 (2013).
Yousefzadeh, M. J. et al. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience 42, 951–961 (2020).
Khrimian, L. et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 214, 2859–2873 (2017). This study identified osteocalcin as a bone-derived rejuvenating factor in young blood.
Mehdipour, M. et al. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline–albumin. Aging 12, 8790–8819 (2020). This study established neutral blood exchange as a new rejuvenating intervention.
Mehdipour, M. et al. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. Geroscience 43, 1–18 (2020).
Middeldorp, J. et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 73, 1325–1333 (2016).
Boada, M. et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: primary results of the AMBAR Study. Alzheimer’s Dement. 16, 1412–1425 (2020).
Speisman, R. B., Kumar, A., Rani, A., Foster, T. C. & Ormerod, B. K. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav. Immun. 28, 25–43 (2013).
Rhyu, I. J. et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 167, 1239–1248 (2010).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108, 3017–3022 (2011).
Ding, Y.-H. et al. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc. Res. 3, 15–23 (2006).
Casaletto, K. et al. Late‐life physical activity relates to brain tissue synaptic integrity markers in older adults. Alzheimers Dement. 18, 2023–2035 (2022).
Miguel, Z. D. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Neeper, S. A., Góauctemez-Pinilla, F., Choi, J. & Cotman, C. Exercise and brain neurotrophins. Nature 373, 109 (1995).
Wahl, D. et al. Comparing the effects of low-protein and high-carbohydrate diets and caloric restriction on brain aging in mice. Cell Rep. 25, 2234–2243 (2018).
Dal-Pan, A. et al. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS ONE 6, e16581 (2011).
Leclerc, E. et al. The effect of caloric restriction on working memory in healthy non-obese adults. CNS Spectr. 25, 2–8 (2020).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009). This seminal study demonstrates the benefits that caloric restriction has on lifespan and age-associated brain atrophy in rhesus macaques.
Lin, A.-L., Zhang, W., Gao, X. & Watts, L. Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol. Aging 36, 2296–2303 (2015).
Kaur, M., Sharma, S. & Kaur, G. Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology 9, 441–454 (2008).
Lee, J., Seroogy, K. B. & Mattson, M. P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 80, 539–547 (2002).
Morgan, T. E. et al. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 89, 687–699 (1999).
Ferreira-Marques, M. et al. Caloric restriction stimulates autophagy in rat cortical neurons through neuropeptide Y and ghrelin receptors activation. Aging 8, 1470–1484 (2016).
McPherron, A. C., Lawler, A. M. & Lee, S.-J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 22, 260–264 (1999).
Egerman, M. A. et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 22, 164–174 (2015).
Ozek, C., Krolewski, R. C., Buchanan, S. M. & Rubin, L. L. Growth differentiation factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci. Rep. 8, 17293 (2018).
Mayweather, B. A., Buchanan, S. M. & Rubin, L. L. GDF11 expressed in the adult brain negatively regulates hippocampal neurogenesis. Mol. Brain 14, 134 (2021).
Oury, F. et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 155, 228–241 (2013).
Castellano, J. M. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492 (2017).
McLay, R. N., Kimura, M., Banks, W. A. & Kastin, A. J. Granulocyte–macrophage colony-stimulating factor crosses the blood–brain and blood–spinal cord barriers. Brain 120, 2083–2091 (1997).
Boyd, T. D. et al. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers Dis. 21, 507–518 (2010).
Gan, K. J. & Südhof, T. C. Specific factors in blood from young but not old mice directly promote synapse formation and NMDA-receptor recruitment. Proc. Natl Acad. Sci. USA 116, 12524–12533 (2019).
Yang, J. et al. Association of accelerated long-term forgetting and senescence-related blood-borne factors in asymptomatic individuals from families with autosomal dominant Alzheimer’s disease. Alzheimers Res. Ther. 13, 107 (2021).
Kurosu, H. et al. Suppression of aging in mice by the hormone klotho. Science 309, 1829–1833 (2005).
Leon, J. et al. Peripheral elevation of a klotho fragment enhances brain function and resilience in young, aging, and α-synuclein transgenic mice. Cell Rep. 20, 1360–1371 (2017). Systemic administration of the longevity factor α-klotho enhances cognitive function in aged mice and models of neurodegeneration.
Hu, M. C. et al. Renal production, uptake, and handling of circulating αklotho. J. Am. Soc. Nephrol. 27, 79–90 (2016).
Zhu, L. et al. Klotho controls the brain–immune system interface in the choroid plexus. Proc. Natl Acad. Sci. USA 115, E11388–E11396 (2018).
Chow, L. S. et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 18, 273–289 (2022).
Schwarz, A. J., Brasel, J. A., Hintz, R. L., Mohan, S. & Cooper, D. M. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J. Clin. Endocrinol. Metab. 81, 3492–3497 (1996).
Reinhardt, R. R. & Bondy, C. A. Insulin-like growth factors cross the blood–brain barrier. Endocrinology 135, 1753–1761 (1994).
Carro, E., Nuñez, A., Busiguina, S. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20, 2926–2933 (2000).
Trejo, J. L., Carro, E. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 (2001).
Markowska, A. L., Mooney, M. & Sonntag, W. E. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 87, 559–569 (1998).
Ding, Q., Vaynman, S., Akhavan, M., Ying, Z. & Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140, 823–833 (2006).
Li, X. et al. Cap‐independent translation of GPLD1 enhances markers of brain health in long‐lived mutant and drug‐treated mice. Aging Cell 21, e13685 (2022).
Leiter, O. et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab. 34, 408–423 (2022).
Pohlkamp, T., Wasser, C. R. & Herz, J. Functional roles of the interaction of APP and lipoprotein receptors. Front. Mol. Neurosci. 10, 54 (2017).
Schwarz, M. et al. Potential protective role of apoprotein J (clusterin) in atherogenesis: binding to enzymatically modified low-density lipoprotein reduces fatty acid-mediated cytotoxicity. Thromb. Haemost. 100, 110–118 (2008).
Islam, M. R. et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 3, 1058–1070 (2021). The systemic myokine irisin reduces neuroinflammation and enhances cognitive function in models of AD pathology.
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Lourenco, M. V. et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175 (2019).
Fabel, K. et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812 (2003).
Hayek, L. E. et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 39, 2369–2382 (2019).
Moon, H. Y. et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 24, 332–340 (2016).
Kestin, A. S. et al. Effect of strenuous exercise on platelet activation state and reactivity. Circulation 88, 1502–1511 (2018).
Leiter, O. et al. Exercise-induced activated platelets increase adult hippocampal precursor proliferation and promote neuronal differentiation. Stem Cell Rep. 12, 667–679 (2019).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Reger, M. A. et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 25, 311–314 (2004).
Murray, A. J. et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 30, 4021–4032 (2016).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557 (2017).
Kashiwaya, Y. et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 34, 1530–1539 (2013).
Dani, N. et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell 184, 3056–3074 (2021).
Baruch, K. et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014).
Baird, G. S. et al. Age-dependent changes in the cerebrospinal fluid proteome by slow off-rate modified aptamer array. Am. J. Pathol. 180, 446–456 (2012).
Silva-Vargas, V., Maldonado-Soto, A. R., Mizrak, D., Codega, P. & Doetsch, F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19, 643–652 (2016).
Iram, T. et al. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature 605, 509–515 (2022).
Acknowledgements
This work was supported by the Simons Foundation (S.A.V.), the Larry L. Hillblom Foundation (G.B.) and the National Institute on Aging (AG064823 (A.B.S.), AG077770 (S.A.V.), AG067740 (S.A.V.)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Fernanda De Felice and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bieri, G., Schroer, A.B. & Villeda, S.A. Blood-to-brain communication in aging and rejuvenation. Nat Neurosci 26, 379–393 (2023). https://doi.org/10.1038/s41593-022-01238-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01238-8
This article is cited by
-
Spatial transcriptomic clocks reveal cell proximity effects in brain ageing
Nature (2025)
-
Antiageing strategy for neurodegenerative diseases: from mechanisms to clinical advances
Signal Transduction and Targeted Therapy (2025)
-
A primary cilia–autophagy axis in hippocampal neurons is essential to maintain cognitive resilience
Nature Aging (2025)
-
Halt aging? — functional HSCs lead the way
Cell Research (2025)
-
Calorie restriction mimetics against aging and inflammation
Biogerontology (2025)