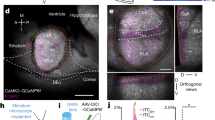
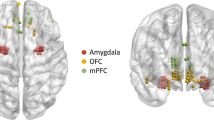
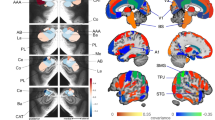
Abstract
The amygdala attributes valence and emotional salience to environmental stimuli and regulates how these stimuli affect behavior. Within the amygdala, a distinct class of evolutionarily conserved neurons form the intercalated cell (ITC) clusters, mainly located around the boundaries of the lateral and basal nuclei. Here, we review the anatomical, physiological and molecular characteristics of ITCs, and detail the organization of ITC clusters and their connectivity with one another and other brain regions. We describe how ITCs undergo experience-dependent plasticity and discuss emerging evidence demonstrating how ITCs are innervated and functionally regulated by neuromodulatory systems. We summarize recent findings showing that experience alters the balance of activity between different ITC clusters, thereby determining prevailing behavioral output. Finally, we propose a model in which ITCs form a key system for integrating divergent inputs and orchestrating brain-wide circuits to generate behavioral states attuned to current environmental circumstances and internal needs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data sharing is not applicable—no new data were generated.
References
Duvarci, S. & Pare, D. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980 (2014).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Grundemann, J. & Luthi, A. Ensemble coding in amygdala circuits for associative learning. Curr. Opin. Neurobiol. 35, 200–206 (2015).
Crosby, E. C. & Humphrey, T. Studies of the vertebrate telencephalon. II. The nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. J. Comp. Neurol. 74, 309–352 (1941).
Millhouse, O. E. The intercalated cells of the amygdala. J. Comp. Neurol. 247, 246–271 (1986).
Palomares-Castillo, E. et al. The intercalated paracapsular islands as a module for integration of signals regulating anxiety in the amygdala. Brain Res. 1476, 211–234 (2012).
Völsch, M. Zur vergleichenden Anatomie des Mandelkerns und seiner Nachbargebilde. Archiv. für. Mikroskopische Anat. 76, 373–523 (1910).
Johnston, J. B. Further contributions to the study of the evolution of the forebrain. J. Comp. Neurol. 35, 337–481 (1923).
Herry, C. et al. Neuronal circuits of fear extinction. Eur. J. Neurosci. 31, 599–612 (2010).
Pape, H. C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 (2010).
Singewald, N. & Holmes, A. Rodent models of impaired fear extinction. Psychopharmacol. 236, 21–32 (2019).
Maren, S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron 70, 830–845 (2011).
Pare, D., Quirk, G. J. & Ledoux, J. E. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 92, 1–9 (2004).
Likhtik, E., Popa, D., Apergis-Schoute, J., Fidacaro, G. A. & Pare, D. Amygdala intercalated neurons are required for expression of fear extinction. Nature 454, 642–645 (2008). By chemically lesioning the medial ITCs after training, this study provided causal evidence for a critical role for ITCs in expression of fear extinction.
Berretta, S., Pantazopoulos, H., Caldera, M., Pantazopoulos, P. & Paré, D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132, 943–953 (2005).
Amir, A., Amano, T. & Pare, D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J. Neurophysiol. 105, 3054–3066 (2011).
Milad, M. R. & Quirk, G. J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74 (2002).
Do-Monte, F. H., Manzano-Nieves, G., Quinones-Laracuente, K., Ramos-Medina, L. & Quirk, G. J. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 35, 3607–3615 (2015).
Bukalo, O. et al. Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci. Adv. 1, e1500251 (2015).
Ehrlich, I. et al. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771 (2009).
Hefner, K. et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J. Neurosci. 28, 8074–8085 (2008).
Busti, D. et al. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J. Neurosci. 31, 5131–5144 (2011). This study demonstrated differential engagement of ITCdm and ITCvm clusters in high and low fear states suggesting a new framework for their role in fear expression and extinction.
Huang, C. C., Chen, C. C., Liang, Y. C. & Hsu, K. S. Long-term potentiation at excitatory synaptic inputs to the intercalated cell masses of the amygdala. Int. J. Neuropsychopharmacol. 17, 1233–1242 (2014).
Asede, D., Bosch, D., Luthi, A., Ferraguti, F. & Ehrlich, I. Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron 86, 541–554 (2015). This study showed that neurons in the ITCdm cluster receive sensory inputs and are part of fear learning-modulated feedforward and feedback inhibitory circuits controlling amygdala input and output nuclei.
Hagihara, K. M. et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nature 594, 403–407 (2021). This study revealed opposing functional roles for ITCdm and ITCvm clusters and provided evidence that through mutual intercluster inhibition, ITCs enable switching between high and low fear states via connections to distinct amygdala–cortical pathways.
Royer, S., Martina, M. & Paré, D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J. Neurosci. 19, 10575–10583 (1999).
Marowsky, A., Yanagawa, Y., Obata, K. & Vogt, K. E. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48, 1025–1037 (2005). This study identified ITCs as a substrate for DA-induced amygdala disinhibition via D1 receptor-dependent hyperpolarization.
Paré, D. & Smith, Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57, 1077–1090 (1993).
McDonald, A. J. & Augustine, J. R. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience 52, 281–294 (1993).
Zikopoulos, B., John, Y. J., Garcia-Cabezas, M. A., Bunce, J. G. & Barbas, H. The intercalated nuclear complex of the primate amygdala. Neuroscience 330, 267–290 (2016).
Braak, H. & Braak, E. Neuronal types in the basolateral amygdaloid nuclei of man. Brain Res. Bull. 11, 349–365 (1983).
Urban, S. & Yilmazer-Hanke, D. M. The pigmentarchitectonic divisions and neuronal types of the central nucleus and intercalated masses of the human amygdala. J. Hirnforsch. 39, 311–319 (1999).
Collins, D. R. & Paré, D. Spontaneous and evoked activity of intercalated amygdala neurons. Eur. J. Neurosci. 11, 3441–3448 (1999).
Asede, D., Doddapaneni, D. & Bolton, M. M. Amygdala intercalated cells: gate keepers and conveyors of internal state to the circuits of emotion. J. Neurosci. 42, 9098–9109 (2022).
Fuxe, K. et al. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation. Neuroscience 119, 733–746 (2003).
Manko, M., Geracitano, R. & Capogna, M. Functional connectivity of the main intercalated nucleus of the mouse amygdala. J. Physiol. 589, 1911–1925 (2011).
Gregoriou, G. C., Kissiwaa, S. A., Patel, S. D. & Bagley, E. E. Dopamine and opioids inhibit synaptic outputs of the main island of the intercalated neurons of the amygdala. Eur. J. Neurosci. 50, 2065–2074 (2019).
Biggs, L. M. & Meredith, M. Functional connectivity of intercalated nucleus with medial amygdala: a circuit relevant for chemosignal processing. IBRO Neurosci. Rep. 12, 170–181 (2022).
Aksoy-Aksel, A., Gall, A., Seewald, A., Ferraguti, F. & Ehrlich, I. Midbrain dopaminergic inputs gate amygdala intercalated cell clusters by distinct and cooperative mechanisms in male mice. eLife 10, e63708 (2021). This study demonstrated multiple mechanisms by which dopaminergic midbrain inputs can shift the activity balance between distinct ITC clusters and showed these effects are affected by extinction learning.
Strobel, C., Marek, R., Gooch, H. M., Sullivan, R. K. & Sah, P. Prefrontal and auditory input to intercalated neurons of the amygdala. Cell Rep. 10, 1435–1442 (2015).
Kwon, O. B. et al. Dopamine regulation of amygdala inhibitory circuits for expression of learned fear. Neuron 88, 378–389 (2015).
Geracitano, R., Kaufmann, W. A., Szabo, G., Ferraguti, F. & Capogna, M. Synaptic heterogeneity between mouse paracapsular intercalated neurons of the amygdala. J. Physiol. 585, 117–134 (2007). This study directly assessed inhibitory synaptic interactions between neighboring ITCs within a cluster (ITCdm), revealing a striking functional heterogeneity in their properties.
Royer, S., Martina, M. & Pare, D. Polarized synaptic interactions between intercalated neurons of the amygdala. J. Neurophysiol. 83, 3509–3518 (2000).
Kaoru, T. et al. Molecular characterization of the intercalated cell masses of the amygdala: implications for the relationship with the striatum. Neuroscience 166, 220–230 (2010). This study uncovered parallels in ontogenetic origin and molecular markers between ITCs and striatal medium spiny neurons.
Kuerbitz, J. et al. Temporally distinct roles for the zinc finger transcription factor Sp8 in the generation and migration of dorsal lateral ganglionic eminence (dLGE)-derived neuronal subtypes in the mouse. Cereb. Cortex 31, 1744–1762 (2021).
Geng, H. Y. et al. Erbb4 deletion from medium spiny neurons of the nucleus accumbens core induces schizophrenia-like behaviors via elevated GABAA receptor α1 subunit expression. J. Neurosci. 37, 7450–7464 (2017).
Asede, D., Okoh, J., Ali, S., Doddapaneni, D. & Bolton, M. M. Deletion of ErbB4 disrupts synaptic transmission and long-term potentiation of thalamic input to amygdalar medial paracapsular intercalated cells. Front. Synaptic Neurosci. 13, 697110 (2021).
Waclaw, R. R., Ehrman, L. A., Pierani, A. & Campbell, K. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J. Neurosci. 30, 6944–6953 (2010).
Kuerbitz, J. et al. Loss of intercalated cells (ITCs) in the mouse amygdala of Tshz1 mutants correlates with fear, depression, and social interaction phenotypes. J. Neurosci. 38, 1160–1177 (2018). This study linked developmental disruption and loss of ITCs to not only fear extinction deficits, but also depression-like and impaired social behaviors.
Nitecka, L. & Ben-Ari, Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J. Comp. Neurol. 266, 45–55 (1987).
Pare, D. & Smith, Y. GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. J. Comp. Neurol. 344, 33–49 (1994).
Pitkanen, A. & Amaral, D. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J. Neurosci. 14, 2200–2224 (1994).
Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W. & Sperk, G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850 (2000).
Marowsky, A., Fritschy, J. M. & Vogt, K. E. Functional mapping of GABAA receptor subtypes in the amygdala. Eur. J. Neurosci. 20, 1281–1289 (2004).
Geracitano, R., Fischer, D., Kasugai, Y., Ferraguti, F. & Capogna, M. Functional expression of the GABAA receptor alpha2 and alpha3 subunits at synapses between intercalated medial paracapsular neurons of mouse amygdala. Front. Neural Circuits 6, 32 (2012).
Sperk, G. et al. Immunohistochemical distribution of 10 GABAA receptor subunits in the forebrain of the rhesus monkey Macaca mulatta. J. Comp. Neurol. 528, 2551–2568 (2020).
Marowsky, A. & Vogt, K. E. Delta-subunit-containing GABAA-receptors mediate tonic inhibition in paracapsular cells of the mouse amygdala. Front. Neural Circuits 8, 27 (2014).
Mar, L., Yang, F. C. & Ma, Q. Genetic marking and characterization of Tac2-expressing neurons in the central and peripheral nervous system. Mol. Brain 5, 3 (2012).
Poulin, J. F., Chevalier, B., Laforest, S. & Drolet, G. Enkephalinergic afferents of the centromedial amygdala in the rat. J. Comp. Neurol. 496, 859–876 (2006).
Winters, B. L. et al. Endogenous opioids regulate moment-to-moment neuronal communication and excitability. Nat. Commun. 8, 14611 (2017). This study showed that endogenous opioid release by ITCs themselves upon moderate activity can regulate their input strength and excitability.
Wood, J. et al. Structure and function of the amygdaloid NPY system: NPY Y2 receptors regulate excitatory and inhibitory synaptic transmission in the centromedial amygdala. Brain Struct. Funct. 221, 3373–3391 (2016).
van den Burg, E. H. & Stoop, R. Neuropeptide signalling in the central nucleus of the amygdala. Cell Tissue Res. 375, 93–101 (2019).
Hajos, N. Interneuron types and their circuits in the basolateral amygdala. Front. Neural Circuits 15, 687257 (2021).
Capogna, M. GABAergic cell type diversity in the basolateral amygdala. Curr. Opin. Neurobiol. 26C, 110–116 (2014).
Strobel, C., Sullivan, R. K. P., Stratton, P. & Sah, P. Calcium signalling in medial intercalated cell dendrites and spines. J. Physiol. 595, 5653–5669 (2017).
Dabrowska, J. & Rainnie, D. G. Expression and distribution of Kv4 potassium channel subunits and potassium channel interacting proteins in subpopulations of interneurons in the basolateral amygdala. Neuroscience 171, 721–733 (2010).
Jacobsen, K. X., Hoistad, M., Staines, W. A. & Fuxe, K. The distribution of dopamine D1 receptor and mu-opioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience 141, 2007–2018 (2006).
Hill, J. E. & Gasser, P. J. Organic cation transporter 3 is densely expressed in the intercalated cell groups of the amygdala: anatomical evidence for a stress hormone-sensitive dopamine clearance system. J. Chem. Neuroanat. 52, 36–43 (2013).
Pinto, A. & Sesack, S. R. Ultrastructural analysis of prefrontal cortical inputs to the rat amygdala: spatial relationships to presumed dopamine axons and D1 and D2 receptors. Brain Struct. Funct. 213, 159–175 (2008).
de la Mora, M. P., Gallegos-Cari, A., Arizmendi-Garcia, Y., Marcellino, D. & Fuxe, K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: structural and functional analysis. Prog. Neurobiol. 90, 198–216 (2010).
Yilmazer-Hanke, D. M. Chapter 22 - Amygdala. in The Human Nervous System (Third Edition) (eds. J. K. Mai et al.) 759–834 (Academic Press, 2012).
O’Leary, T. P. et al. Extensive and spatially variable within-cell-type heterogeneity across the basolateral amygdala. eLife 9, e59003 (2020).
Royer, S., Martina, M. & Pare, D. Bistable behavior of inhibitory neurons controlling impulse traffic through the amygdala: role of a slowly deinactivating K+ current. J. Neurosci. 20, 9034–9039 (2000).
Asede, D. et al. Apical intercalated cell cluster: A distinct sensory regulator in the amygdala. Cell Rep. 35, 109151 (2021). This study identified the ITCap cluster as an inhibitory relay between multiple cortical and thalamic inputs and outputs to lateral amygdala and dorsal striatum.
Royer, S. & Paré, D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature 422, 518–522 (2003). This study showed that activity-dependent plasticity of ITC glutamatergic inputs lead to coordinated changes in synaptic strength that could serve to maintain overall synaptic weights.
Jüngling, K. et al. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59, 298–310 (2008). This study suggests that the anxiolytic and extinction-enhancing effects of endogenous NPS are mediated by modulation of inputs to ITCs.
Blaesse, P. et al. μ-opioid receptor-mediated inhibition of intercalated neurons and effect on synaptic transmission to the central amygdala. J. Neurosci. 35, 7317–7325 (2015).
Rajbhandari, A. K. et al. A basomedial amygdala to intercalated cells microcircuit expressing PACAP and its receptor PAC1 regulates contextual fear. J. Neurosci. 41, 3446–3461 (2021). This study revealed a sexually dimorphic function of a basomedial amygdala to ITC circuit that co-releases PACAP in regulating fear generalization and extinction.
Silberman, Y., Ariwodola, O. J., Chappell, A. M., Yorgason, J. T. & Weiner, J. L. Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology 35, 1886–1896 (2010).
Gregoriou, G. C., Patel, S. D., Pyne, S., Winters, B. L. & Bagley, E. E. Opioid withdrawal abruptly disrupts amygdala circuit function by reducing peptide actions. J. Neurosci. 43, 1668–1681 (2023).
Senn, V. et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014).
Tovote, P. et al. Midbrain circuits for defensive behaviour. Nature 534, 206–212 (2016).
Ghashghaei, H. T. & Barbas, H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115, 1261–1279 (2002).
Moga, M. M. & Gray, T. S. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J. Comp. Neurol. 241, 275–284 (1985).
Stern, D. B., Wilke, A. & Root, C. M. Anatomical connectivity of the intercalated cells of the amygdala. eNeuro 10, ENEURO.0238-23.2023 (2023).
Milad, M. R., Vidal-Gonzalez, I. & Quirk, G. J. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav. Neurosci. 118, 389–394 (2004).
McDonald, A. J., Mascagni, F. & Guo, L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71, 55–75 (1996).
Vertes, R. P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58 (2004).
Pinard, C. R., Mascagni, F. & McDonald, A. J. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205, 112–124 (2012).
Dobi, A. et al. Neural substrates for the distinct effects of presynaptic group III metabotropic glutamate receptors on extinction of contextual fear conditioning in mice. Neuropharmacology 66, 274–289 (2013).
Freedman, L. J., Insel, T. R. & Smith, Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J. Comp. Neurol. 421, 172–188 (2000).
Zikopoulos, B., Hoistad, M., John, Y. & Barbas, H. Posterior orbitofrontal and anterior cingulate pathways to the amygdala target inhibitory and excitatory systems with opposite functions. J. Neurosci. 37, 5051–5064 (2017).
Cho, J. H., Deisseroth, K. & Bolshakov, V. Y. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80, 1491–1507 (2013).
Adhikari, A. et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527, 179–185 (2015).
Bazelot, M. et al. Hippocampal theta input to the amygdala shapes feedforward inhibition to gate heterosynaptic plasticity. Neuron 87, 1290–1303 (2015).
Tovote, P., Fadok, J. P. & Luthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015).
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010).
Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (2013).
Whittle, N. et al. Central amygdala micro-circuits mediate fear extinction. Nat. Commun. 12, 4156 (2021).
Royer, S. & Paré, D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 115, 455–462 (2002).
Seewald, A. et al. Fear memory retrieval is associated with a reduction in AMPA receptor density at thalamic to amygdala intercalated cell synapses. Front. Synaptic Neurosci. 13, 634558 (2021).
Morozov, A., Sukato, D. & Ito, W. Selective suppression of plasticity in amygdala inputs from temporal association cortex by the external capsule. J. Neurosci. 31, 339–345 (2011).
Skelly, M. J., Chappell, A. M., Ariwodola, O. J. & Weiner, J. L. Behavioral and neurophysiological evidence that lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the acquisition and extinction of fear learning. Neurobiol. Learn Mem. 127, 10–16 (2016).
Skelly, M. J., Ariwodola, O. J. & Weiner, J. L. Fear conditioning selectively disrupts noradrenergic facilitation of GABAergic inhibition in the basolateral amygdala. Neuropharmacology 113, 231–240 (2017).
Amano, T., Unal, C. T. & Pare, D. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 13, 489–494 (2010). This study demonstrated extinction-driven synaptic plasticity of ITCs and showed that infralimbic-dependent potentiation of BLA inputs to ITCs drives increased inhibition of fear output neurons in CeA.
An, B. et al. Amount of fear extinction changes its underlying mechanisms. eLife 6, e25224 (2017).
Knapska, E. & Maren, S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 16, 486–493 (2009).
Whittle, N., Hauschild, M., Lubec, G., Holmes, A. & Singewald, N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J. Neurosci. 30, 13586–13596 (2010).
Dorofeikova, M. et al. Effects of footshock stress on social behavior and neuronal activation in the medial prefrontal cortex and amygdala of male and female mice. PLoS ONE 18, e0281388 (2023).
Gouty, S., Silveira, J. T., Cote, T. E. & Cox, B. M. Aversive stress reduces mu opioid receptor expression in the intercalated nuclei of the rat amygdala. Cell Mol. Neurobiol. 41, 1119–1129 (2021).
Becker, J. A. et al. Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology 39, 2049–2060 (2014).
Zhang, X., Kiyokawa, Y. & Takeuchi, Y. Mapping of c-Fos expression in the medial amygdala following social buffering in male rats. Behav. Brain Res 422, 113746 (2022).
Minami, S., Kiyokawa, Y. & Takeuchi, Y. The lateral intercalated cell mass of the amygdala is activated during social buffering of conditioned fear responses in male rats. Behav. Brain Res. 372, 112065 (2019).
St. Laurent, R. et al. Intercalated amygdala dysfunction drives extinction deficits in the Sapap3 mouse model of obsessive-compulsive disorder. Biol. Psychiatry https://doi.org/10.1016/j.biopsych.2024.10.021 (2024).
Hagihara, K. M. & Luthi, A. Bidirectional valence coding in amygdala intercalated clusters: a neural substrate for the opponent-process theory of motivation. Neurosci. Res. https://doi.org/10.1016/j.neures.2024.07.003 (2024).
Likhtik, E. & Johansen, J. P. Neuromodulation in circuits of aversive emotional learning. Nat. Neurosci. 22, 1586–1597 (2019).
Bromberg-Martin, E. S., Matsumoto, M. & Hikosaka, O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834 (2010).
Salinas-Hernandez, X. I. et al. Dopamine neurons drive fear extinction learning by signaling the omission of expected aversive outcomes. eLife 7, e38818 (2018).
Lee, J. H., Lee, S. & Kim, J. H. Amygdala circuits for fear memory: a key role for dopamine regulation. Neuroscientist 23, 542–553 (2017).
Asan, E. The catecholaminergic innervation of the rat amygdala. Adv. Anat. Embryol. Cell Biol. 142, 1–118 (1998).
Asan, E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 288, 449–469 (1997).
Ferrazzo, S. et al. Increased anxiety-like behavior following circuit-specific catecholamine denervation in mice. Neurobiol. Dis. 125, 55–66 (2019).
Tritsch, N. X., Ding, J. B. & Sabatini, B. L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266 (2012).
Mingote, S. et al. Functional connectome analysis of dopamine neuron glutamatergic connections in forebrain regions. J. Neurosci. 35, 16259–16271 (2015).
Lutas, A. et al. State-specific gating of salient cues by midbrain dopaminergic input to basal amygdala. Nat. Neurosci. 22, 1820–1833 (2019).
McCall, J. G. et al. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife 6, e18247 (2017).
Roozendaal, B. et al. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol. Learn Mem. 86, 249–255 (2006).
Asan, E., Steinke, M. & Lesch, K. P. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem. Cell Biol. 139, 785–813 (2013).
Linley, S. B., Olucha-Bordonau, F. & Vertes, R. P. Pattern of distribution of serotonergic fibers to the amygdala and extended amygdala in the rat. J. Comp. Neurol. 525, 116–139 (2017).
O’Rourke, H. & Fudge, J. L. Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol. Psychiatry 60, 479–490 (2006).
Poulin, J.-F., Castonguay-Lebel, Z., Laforest, S. & Drolet, G. Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat. J. Comp. Neurol. 506, 943–959 (2008).
Gregoriou, G. C., Patel, S. D., Winters, B. L. & Bagley, E. E. Neprilysin controls the synaptic activity of neuropeptides in the intercalated cells of the amygdala. Mol. Pharmacol. 98, 454–461 (2020).
Clark, S. D. et al. Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J. Comp. Neurol. 519, 1867–1893 (2011).
Neugebauer, V. et al. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 170, 108052 (2020).
Chauveau, F. et al. Prevention of stress-impaired fear extinction through neuropeptide S action in the lateral amygdala. Neuropsychopharmacology 37, 1588–1599 (2012).
Ren, W. et al. Neuropeptide S: a novel regulator of pain-related amygdala plasticity and behaviors. J. Neurophysiol. 110, 1765–1781 (2013).
Medina, G., Ji, G., Grégoire, S. & Neugebauer, V. Nasal application of neuropeptide S inhibits arthritis pain-related behaviors through an action in the amygdala. Mol. Pain. 10, 32 (2014).
Ressler, K. J. et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497 (2011).
Pérez de la Mora, M. et al. Role of the amygdaloid cholecystokinin (CCK)/gastrin-2 receptors and terminal networks in the modulation of anxiety in the rat. Effects of CCK-4 and CCK-8S on anxiety-like behaviour and [3H]GABA release. Eur. J. Neurosci. 26, 3614–3630 (2007).
Narvaez, M. et al. A novel integrative mechanism in anxiolytic behavior induced by galanin 2/neuropeptide Y Y1 receptor interactions on medial paracapsular intercalated amygdala in rats. Front. Cell Neurosci. 12, 119 (2018).
Narvaez, M. et al. Galanin receptor 2-neuropeptide Y Y1 receptor interactions in the amygdala lead to increased anxiolytic actions. Brain Struct. Funct. 220, 2289–2301 (2015).
Izquierdo, A., Brigman, J. L., Radke, A. K., Rudebeck, P. H. & Holmes, A. The neural basis of reversal learning: an updated perspective. Neuroscience 345, 12–26 (2017).
Meins, M. et al. Impaired fear extinction in mice lacking protease nexin-1. Eur. J. Neurosci. 31, 2033–2042 (2010).
Zangrandi, L. et al. Loss of mGluR5 in D1 receptor-expressing neurons improves stress coping. Int. J. Mol. Sci. 22, 7826 (2021).
Bienvenu, T. C. et al. Large intercalated neurons of amygdala relay noxious sensory information. J. Neurosci. 35, 2044–2057 (2015).
Zussy, C. et al. Dynamic modulation of inflammatory pain-related affective and sensory symptoms by optical control of amygdala metabotropic glutamate receptor 4. Mol. Psychiatry 23, 509–520 (2018).
Burke, D. A., Rotstein, H. G. & Alvarez, V. A. Striatal local circuitry: a new framework for lateral inhibition. Neuron 96, 267–284 (2017).
Bariselli, S., Fobbs, W. C., Creed, M. C. & Kravitz, A. V. A competitive model for striatal action selection. Brain Res. 1713, 70–79 (2019).
Klaus, A., Alves da Silva, J. & Costa, R. M. What, if, and when to move: basal ganglia circuits and self-paced action initiation. Annu. Rev. Neurosci. 42, 459–483 (2019).
Lanciego, J. L., Luquin, N. & Obeso, J. A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2, a009621 (2012).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.A.-A., F.F., A.H., A.L. and I.E. Supervision: I.E. Visualization: A.A.-A., F.F. and I.E. All authors participated in writing the original draft and in reviewing and editing the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Pankaj Sah and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aksoy-Aksel, A., Ferraguti, F., Holmes, A. et al. Amygdala intercalated cells form an evolutionarily conserved system orchestrating brain networks. Nat Neurosci 28, 234–247 (2025). https://doi.org/10.1038/s41593-024-01836-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-024-01836-8