Abstract
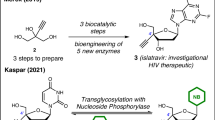
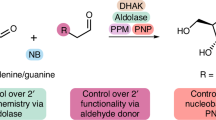
Nucleoside analogs are valuable commodities in the development of antisense oligonucleotides or as stand-alone antiviral and anticancer therapies. Syntheses of nucleoside analogs are typically challenged by a reliance on chiral pool starting materials and inefficient synthetic routes that are not readily amenable to diversification. The novel methodology described in this protocol addresses several longstanding challenges in nucleoside analog synthesis by enabling flexible and selective access to nucleoside analogs possessing variable nucleobase substitution, D- or L-configuration, selective protection of C3′/C5′ alcohols and C2′ or C4′ derivatizations. This protocol provides direct access to C3′/C5′ protected nucleoside analogs in three steps from simple, achiral starting materials and is described on both research (2.8 g) and process (30 g) scales for the synthesis of C3′/C5′-acetonide protected uridine. Using this protocol, proline catalyzes the fluorination of simple heteroaryl-substituted aldehyde starting materials, which are then directly engaged in a one-pot enantioselective aldol reaction with a dioxanone. Reduction, followed by intramolecular annulative fluoride displacement, forges the nucleoside analog. The three-step parent protocol can be completed in ~5 d by using simple mix-and-stir reaction procedures and standard column chromatographic purification techniques.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data described in Anticipated results were obtained from the materials prepared by using the protocol described herein and are concordant with the data that we have previously reported for compounds 4a/b, 5a/b and 1 (ref. 9).
References
Shelton, J. et al. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem. Rev. 116, 14379–14455 (2016).
First Oral Antiviral for COVID-19, Lageviro (molnupiravir), approved by MHRA. Medicines and Healthcare products Regulatory Agency (MHRA) (Press release). 4 November 2021. Retrieved 23 November 2021. https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra
Merck and Ridgeback’s Molnupiravir, an Oral COVID-19 Antiviral Medicine, Receives First Authorization in the World. Merck & Co. (Press release). 4 November 2021. Retrieved 23 November 2021. https://www.merck.com/news/merck-and-ridgebacks-molnupiravir-an-oral-covid-19-antiviral-medicine-receives-first-authorization-in-the-world/
Quemener, A. M. et al. The powerful world of antisense oligonucleotides: from bench to bedside. Wiley Interdiscip. Rev. RNA 11, e1594 (2020).
Kim, J. et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381, 1644–1652 (2019).
McLaughlin, M. et al. Enantioselective synthesis of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) via enzymatic desymmetrization. Org. Lett. 19, 926–929 (2017).
Peifer, M., Berger, R., Shurtleff, V. W., Conrad, J. C. & MacMillan, D. W. C. A general and enantioselective approach to pentoses: a rapid synthesis of PSI-6130, the nucleoside core of sofosbuvir. J. Am. Chem. Soc. 136, 5900–5903 (2014).
Chun, B. K. et al. Methods for treating Filoviridae virus infections. US Patent 9724360 B2 (2017).
Meanwell, M. et al. A short de novo synthesis of nucleoside analogs. Science 369, 725–730 (2020).
Miller, G. J. Unifying the synthesis of nucleoside analogs. Science 369, 623 (2020).
Meanwell, M. et al. Diversity-oriented synthesis of glycomimetics. Commun. Chem. 4, 96 (2021).
Seth, P. P. & Swayze, E. E. 6-Disubstituted or unsaturated bicyclic nucleic acid analogs. US Patent 8278283 B2 (2008).
Acknowledgements
The authors acknowledge Michael Smith Health Research BC for financial support in the form of a Research Trainee Award (E.K.D.). R.B. acknowledges support from the Canadian Glycomics Network (Strategic Initiatives Grant CD-81); the Consortium de Recherche Biopharmaceutique (CQDM Quantum Leap Grant); Merck & Co., Inc.; and the Natural Sciences and Engineering Research Council (NSERC) of Canada (Discovery Grant, RGPIN-2019-064680).
Author information
Authors and Affiliations
Contributions
E.K.D. optimized the research-scale protocol and wrote the manuscript and supporting information. D.A.P. optimized the process-scale protocol and wrote the associated procedures. M.M. carried out initial discovery efforts for this protocol. M.B.N. optimized the synthesis of the aldehyde precursors. S.M.S., L.-C.C. and R.B. supervised the project.
Corresponding author
Ethics declarations
Competing interests
Simon Fraser University and Merck & Co., Inc. have filed a patent application describing the synthesis of nucleoside analogs via the process presented in this manuscript—U.S. provisional patent application No. 62/994,349.
Peer review
Peer review information
Nature Protocols thanks Yongguirobin Chi and Henning Jacob Jessen for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Meanwell, M. et al. Science 369, 725–730 (2020): https://www.science.org/doi/10.1126/science.abb3231
Supplementary information
Supplementary Information
Supplementary Fig. 1; Supplementary Procedures, Troubleshooting table and Anticipated results; and Supplementary Figs. 2–7 (spectra).
Rights and permissions
About this article
Cite this article
Davison, E.K., Petrone, D.A., Meanwell, M. et al. Practical and concise synthesis of nucleoside analogs. Nat Protoc 17, 2008–2024 (2022). https://doi.org/10.1038/s41596-022-00705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00705-7
This article is cited by
-
Unmasking the halide effect in diastereoselective Grignard reactions applied to C4´ modified nucleoside synthesis
Nature Communications (2025)
-
An enantioselective and modular platform for C4ʹ-modified nucleoside analogue synthesis enabled by intramolecular trans-acetalizations
Nature Communications (2024)