Abstract
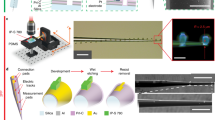
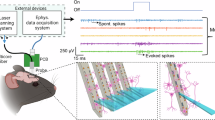
Implantable multifunctional probes have transformed neuroscience research, offering access to multifaceted brain activity that was previously unattainable. Typically, simultaneous access to both optical and electrical signals requires separate probes, while their integration into a single device can result in the emergence of photogenerated electrical artifacts, affecting the quality of high-frequency neural recordings. Among the nontrivial strategies aimed at the realization of an implantable multifunctional interface, the integration of optical and electrical capabilities on a single, minimally invasive, tapered optical fiber probe has been recently demonstrated using fibertrodes. Fibertrodes require the application of a set of planar microfabrication techniques to a nonplanar system with low and nonconstant curvature radius. Here we develop a process based on multiple conformal depositions, nonplanar two-photon lithography and chemical wet etching steps to obtain metallic patterns on the highly curved surface of the fiber taper. We detail the manufacturing, encapsulation and back end of the fibertrodes. The design of the probe can be adapted for different experimental requirements. Using the optical setup design, it is possible to perform angle selective light coupling with the fibertrodes and their implantation and use in vivo. The fabrication of fibertrodes is estimated to require 5–9 d. Nonetheless, due to the high scalability of a large part of the protocol, the manufacture of multiple fibertrodes simultaneously substantially reduces the required time for each probe. The procedure is suitable for users with expertise in microfabrication of electronics and neural recordings.
Key points
-
The procedure covers microfabrication approaches including the conformal deposition of metallic and dielectric thin films, nonplanar two-photon lithography, wet etching and focused ion beam milling/deposition.
-
The integration of electrical and optical signals within a single device enables the optical control of neural activity and the recording of simultaneous electrophysiological readout in mice. Alternative approaches include the use of flexible electronics, micro light-emitting diodes, multifunctional polymeric optical fibers and silica-based arrays.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
Data availability
The authors declare that the main data discussed in this protocol are available in the supporting primary research paper (https://doi.org/10.1038/s41563-022-01272-8) and are available in the Zenodo repository via https://doi.org/10.5281/zenodo.6477861 (ref. 59).
Code availability
The scripts related to the Monte Carlo simulations have been published in ref. 21 and are available in the Zenodo repository via https://doi.org/10.5281/zenodo.6477861 (ref. 59). The driving software of the custom TPP setup is available at Zenodo via https://doi.org/10.5281/zenodo.12773241 (ref. 60), along with a 3D computer-aided design (CAD) model and a detailed list of all the elements of the system at Zenodo via https://doi.org/10.5281/zenodo.6536237 (ref. 28).
References
Grosenick, L., Marshel, J. H. & Deisseroth, K. Closed-loop and activity-guided optogenetic control. Neuron 86, 106–139 (2015).
Kim, C., Jeong, J. & Kim, S. J. Recent progress on non‐conventional microfabricated probes for the chronic recording of cortical neural activity. Sensors 19, 1069 (2019).
Imfeld, K. et al. Large-scale, high-resolution data acquisition system for extracellular recording of electrophysiological activity. IEEE Trans. Biomed. Eng. 55, 2064–2073 (2008).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Durand, S. et al. Acute head-fixed recordings in awake mice with multiple Neuropixels probes. Nat. Protoc. 18, 424–457 (2023).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Lu, L. et al. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proc. Natl Acad. Sci. USA 115, E1374–E1383 (2018).
Wu, F. et al. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. J. Neural Eng. 10, 56012 (2013).
McAlinden, N. et al. Multisite microLED optrode array for neural interfacing. Neurophotonics 6, 035010 (2019).
Yang, Y. et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 24, 1035–1045 (2021).
Yang, Y. et al. Preparation and use of wireless reprogrammable multilateral optogenetic devices for behavioral neuroscience. Nat. Protoc. 17, 1073–1096 (2022).
Du, J., Roukes, M. L. & Masmanidis, S. C. Dual-side and three-dimensional microelectrode arrays fabricated from ultra-thin silicon substrates. J. Micromech. Microeng. 19, 075008 (2009).
Shin, S. et al. Novel four-sided neural probe fabricated by a thermal lamination process of polymer films. J. Neurosci. Methods 278, 25–35 (2017).
Stieglitz, T. & Gross, M. Flexible BIOMEMS with electrode arrangements on front and back side as key component in neural prostheses and biohybrid systems. Sens. Actuators B 83, 8–14 (2002).
Seymour, J. P., Langhals, N. B., Anderson, D. J. & Kipke, D. R. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomed. Microdevices 13, 441–451 (2011).
Zhao, Z. et al. Nanoelectronic coating enabled versatile multifunctional neural probes. Nano Lett. 17, 4588–4595 (2017).
Wu, F. et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88, 1136–1148 (2015).
Gasemi, P., Veladi, H. & Shahabi, P. Flexible deep brain neural probes based on a parylene tube structure related content flexible poly(methyl methacrylate)-based neural probe: an affordable implementation. J. Micromech. Microeng. 28, 015012 (2018).
Tamaki, S. et al. Flexible tube-shaped neural probe for recording and optical stimulation of neurons at arbitrary depths. Sens. Mater. 27, 507–523 (2015).
Wang, M.-H. et al. Flexible cylindrical neural probe with graphene enhanced conductive polymer for multi-mode BCI applications. In 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems, 502–505 (IEEE, 2017).
Spagnolo, B. et al. Tapered fibertrodes for optoelectrical neural interfacing in small brain volumes with reduced artefacts. Nat. Mater. 21, 826–835 (2022).
Pisanello, F. et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 20, 1180–1188 (2017).
Pisano, F. et al. Depth-resolved fiber photometry with a single tapered optical fiber implant. Nat. Methods 16, 1185–1192 (2019).
Pisanello, M. et al. Tailoring light delivery for optogenetics by modal demultiplexing in tapered optical fibers. Sci. Rep. 8, 1–11 (2018).
Balena, A. et al. Two-photon fluorescence-assisted laser ablation of non-planar metal surfaces: fabrication of optical apertures on tapered fibers for optical neural interfaces. Opt. Express 28, 21368–21381 (2020).
Bianco, M. et al. Orthogonalization of far-field detection in tapered optical fibers for depth-selective fiber photometry in brain tissue. APL Photonics 7, 26106 (2022).
Yi, L., Hou, B., Zhao, H., Tan, H. Q. & Liu, X. A double-tapered fibre array for pixel-dense gamma-ray imaging. Nat. Photon. 17, 494–500 (2023).
Pisanello, M. et al. An open source three-mirror laser scanning holographic two-photon lithography system. PLoS ONE 17, e0265678–e0265678 (2022).
Snyder, A. W. & Love, J. D. Optical Waveguide Theory (Springer, 1983).
Pisanello, M. et al. Modal demultiplexing properties of tapered and nanostructured optical fibers for in vivo optogenetic control of neural activity. Biomed. Opt. Express 6, 4014–4026 (2015).
Mohanty, A. et al. Reconfigurable nanophotonic silicon probes for sub-millisecond deep-brain optical stimulation. Nat. Biomed. Eng. 4, 223–231 (2020).
Park, S. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 20, 612–619 (2017).
Pisanello, F. et al. Multipoint-emitting optical fibers for spatially addressable in vivo optogenetics. Neuron 82, 1245–1254 (2014).
Sileo, L. et al. Tapered fibers combined with a multi-electrode array for optogenetics in mouse medial prefrontal cortex. Front. Neurosci. https://doi.org/10.3389/fnins.2018.00771 (2018).
Lee, Y. et al. Selectively micro-patternable fibers via in-fiber photolithography. ACS Cent. Sci. 6, 2319–2325 (2020).
Jiang, S. et al. Spatially expandable fiber-based probes as a multifunctional deep brain interface. Nat. Commun. 11, 6115 (2020).
Kozai, T. D. Y. & Vazquez, A. L. Photoelectric artefact from optogenetics and imaging on microelectrodes and bioelectronics: new challenges and opportunities. J. Mater. Chem. 3, 4965–4978 (2015).
Kampasi, K. et al. Dual color optogenetic control of neural populations using low-noise, multishank optoelectrodes. Microsyst. Nanoeng. 4, 10 (2018).
Sridharan, S. et al. High-performance microbial opsins for spatially and temporally precise perturbations of large neuronal networks. Neuron 110, 1139–1155.e6 (2022).
Pisano, F. et al. Focused ion beam nanomachining of tapered optical fibers for patterned light delivery. Microelectron. Eng. 145, 41–49 (2018).
Kilic, T. et al. Zwitterionic polymer electroplating facilitates the preparation of electrode surfaces for biosensing. Adv. Mater. 34, 2107892 (2022).
Deng, M. et al. Electrochemical deposition of polypyrrole/graphene oxide composite on microelectrodes towards tuning the electrochemical properties of neural probes. Sens. Actuators B 158, 176–184 (2011).
Ruggiero, A. et al. Two-photon polymerization lithography enabling the fabrication of PEDOT:PSS 3D structures for bioelectronic applications. Chem. Commun. 58, 9790–9793 (2022).
Balena, A., Bianco, M., Pisanello, F. & De Vittorio, M. Recent advances on high‐speed and holographic two‐photon direct laser writing. Adv. Funct. Mater. https://doi.org/10.1002/adfm.202211773 (2023).
Beltramo, R. et al. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat. Neurosci. 16, 227–234 (2013).
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Bianco, M. et al. Comparative study of autofluorescence in flat and tapered optical fibers towards application in depth-resolved fluorescence lifetime photometry in brain tissue. Biomed. Opt. Express 12, 993–1009 (2021).
Balena, A. et al. Tapered fibers for optogenetics: Gaining spatial resolution in deep brain regions by exploiting angle-selective light injection systems. In International Conference on Transparent Optical Networks, 1–7 (IEEE, 2019).
Sileo, L., Pisanello, M., De Vittorio, M. & Pisanello, F. Fabrication of multipoint light emitting optical fibers for optogenetics. Neurophoton. Optogenet. https://doi.org/10.1117/12.2075819 (2015).
Sparta, D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2012).
Žukauskas, A. et al. Black silicon: substrate for laser 3D micro/nano-polymerization. Opt. Express 21, 6901–6909 (2013).
Paxinos, G. & Franklin, K. B. J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates (Elsevier, 2019).
McSweeney, C. & Mao, Y. Applying stereotactic injection technique to study genetic effects on animal behaviors. J. Vis. Exp. https://doi.org/10.3791/52653 (2015).
Lewis, C. M. et al. Recording quality is systematically related to electrode impedance. Adv. Health. Mater. https://doi.org/10.1002/adhm.202303401 (2024).
Fan, B., Wolfrum, B. & Robinson, J. T. Impedance scaling for gold and platinum microelectrodes. J. Neural Eng. 18, 056025 (2021).
Boehler, C., Vieira, D. M., Egert, U. & Asplund, M. NanoPt—a nanostructured electrode coating for neural recording and microstimulation. ACS Appl. Mater. Interfaces 12, 14855–14865 (2020).
Horch, K. & Kipke, D. Neuroprosthetics Vol. 8 (World Scientific, 2017).
Zheng, D. et al. Toward plasmonic neural probes: SERS detection of neurotransmitters through gold‐nanoislands‐decorated tapered optical fibers with sub‐10 nm gaps. Adv. Mater. 35, e2200902 (2023).
Spagnolo, B. et al. Raw data of manuscript ‘Tapered fibertrodes for opto-electrical neural interfacing in small brain volumes with reduced artefacts’. Zenodo https://doi.org/10.5281/zenodo.6477861 (2022).
Pisanello, M. et al. Software - An open source three-mirror laser scanning holographic two-photon lithography system. Zenodo https://doi.org/10.5281/zenodo.12773241 (2022).
Acknowledgements
A.B., M.B., F.Pisano B.S. and F. Pisanello acknowledge funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (#677683). M.P. and M.D.V. acknowledge funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (#692943). M.B., C.M., M.D.V. and F. Pisanello acknowledge funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (#966674). M.B., F.Pisano, M.D.V. and F.Pisanello. acknowledge that this project has received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement no. 101016787. M.D.V. is funded by the US National Institutes of Health (U01NS094190). M.P., F.Pisanello and M.D.V. are funded by the US National Institutes of Health (1UF1NS108177-01). A.B. acknowledges funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement (no. 101106602). A.B., F.Pisanello and M.D.V. also acknowledge funding from the European Union’s Horizon 2020 research and innovation program under a grant agreement (no. 828972). C.M., B.S., F.Pisanello and M.D.V. acknowledge funding from project ‘RAISE (Robotics and AI for Socio-economic Empowerment)’ code ECS00000035 funded by European Union–NextGenerationEU PNRR MUR– M4C2–Investimento 1.5– Avviso ‘Ecosistemi dell’Innovazione’ CUP J33C22001220001. F. Pisano acknowledges funding from PARD 2024 from the University of Padua.
Author information
Authors and Affiliations
Contributions
A.B., M.B. and M.P. designed and implemented the fabrication protocol. M.P. and F.Pisano designed the optical setup for depth-resolved optogenetic stimulation. C.M. and B.S. conceived the in vivo probe implantation routine. A.B. wrote the paper with extensive contributions from M.S.A. and M.B. on the fabrication part, M.P and F.Pisano on the optical setup design part and C.M. and B.S. on the in vivo implantation part. F. Pisanello, M.D.V. and B.L.S. jointly supervised the work. All the authors discussed the results and commented on the manuscript at all stages. All the authors contributed extensively to the work.
Corresponding authors
Ethics declarations
Competing interests
F.Pisanello, M.D.V. and B.L.S. are founders and hold private equity in Optogenix, a company that develops, produces and sells technologies to deliver light into the brain. Tapered fibers commercially available from Optogenix were used as tools in the research. The other authors do not declare any competing interests.
Peer review
Peer review information
Nature Protocols thanks Angelique Park, Seongjun Park and the other, anonymous, reviewer(s) for the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key reference
Spagnolo, B. et al. Tapered fibertrodes for optoelectrical neural interfacing in small brain volumes with reduced artefacts. Nat. Mater. https://doi.org/10.1038/s41563-022-01272-8 (2022)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balena, A., Bianco, M., Andriani, M.S. et al. Fabrication of nonplanar tapered fibers to integrate optical and electrical signals for neural interfaces in vivo. Nat Protoc 20, 1768–1809 (2025). https://doi.org/10.1038/s41596-024-01105-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-024-01105-9
This article is cited by
-
Materials and device strategies to enhance spatiotemporal resolution in bioelectronics
Nature Reviews Materials (2025)