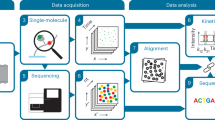
Abstract
Single-molecule techniques are exceptionally well suited for analyzing the complex dynamic behavior of macromolecules involved in fundamental biological processes. Nevertheless, time and cost usually restrict current single-molecule methods to examining a limited number of different samples. At the same time, a broad sequence or chemical space often needs to be investigated to gain a thorough understanding of complex biological phenomena. To address this urgent need, we have developed multiplexed single-molecule characterization at the library scale (MUSCLE), a method that combines single-molecule fluorescence microscopy with next-generation sequencing to enable highly multiplexed observations of complex dynamics on millions of individual molecules spanning thousands of distinct sequences or barcoded entities. In this protocol, we outline the implementation of MUSCLE and present examples from our recent research, such as the sequence-dependent dynamics of Cas9-induced target DNA unwinding and rewinding. This example demonstrates that MUSCLE can be applied to study protein–nucleic acid interactions, going beyond nucleic-acid-only model systems. We detail the sample and library design, high-throughput single-molecule data acquisition, next-generation sequencing, spatial registration of single-molecule fluorescence and sequencing data and downstream data analysis. The ligation-based surface immobilization approach of MUSCLE ensures high clustering efficiency (>40%), increasing throughput and simplifying registration. In addition, MUSCLE includes a 3D-printed flow cell adapter that enables liquid exchange during single-molecule fluorescence microscopy. The complete procedure typically spans 3–4 days and yields a dataset that comprehensively characterizes the dynamic behavior of a library of constructs.
Key points
-
MUSCLE leverages single-molecule fluorescence microscopy and next-generation sequencing to characterize in parallel the dynamic behavior of millions of DNA molecules immobilized on an Illumina flow cell by matching single-molecule traces to the corresponding sequenced clusters.
-
Beyond probing how sequence affects the dynamics of single DNA molecules, this approach can be used to study protein–nucleic acid interactions, as illustrated by the sequence-dependent dynamics of Cas9-induced target DNA unwinding and rewinding.
This is a preview of subscription content, access via your institution
Access options





Similar content being viewed by others
Code availability
The latest version of the MUSCLE data analysis codes can be found at https://github.com/deindllab/MUSCLE, while the current version has been archived in the SciLifeLab Data Repository77.
References
Mohapatra, S., Lin, C.-T., Feng, X. A., Basu, A. & Ha, T. Single-molecule analysis and engineering of DNA motors. Chem. Rev. 120, 36–78 (2020).
Bacic, L., Sabantsev, A. & Deindl, S. Recent advances in single-molecule fluorescence microscopy render structural biology dynamic. Curr. Opin. Struct. Biol. 65, 61–68 (2020).
Hill, F. R., Monachino, E. & van Oijen, A. M. The more the merrier: high-throughput single-molecule techniques. Biochem. Soc. Trans. 45, 759–769 (2017).
Choi, J., Grosely, R., Puglisi, E. V. & Puglisi, J. D. Expanding single-molecule fluorescence spectroscopy to capture complexity in biology. Curr. Opin. Struct. Biol. 58, 233–240 (2019).
Orrit, M., Ha, T. & Sandoghdar, V. Single-molecule optical spectroscopy. Chem. Soc. Rev. 43, 973–976 (2014).
Kulzer, F. & Orrit, M. Single-molecule optics. Annu. Rev. Phys. Chem. 55, 585–611 (2004).
Dangkulwanich, M., Ishibashi, T., Bintu, L. & Bustamante, C. Molecular mechanisms of transcription through single-molecule experiments. Chem. Rev. 114, 3203–3223 (2014).
Zhou, J., Schweikhard, V. & Block, S. M. Single-molecule studies of RNAPII elongation. Biochim. Biophys. Acta 1829, 29–38 (2013).
Michaelis, J. & Treutlein, B. Single-molecule studies of RNA polymerases. Chem. Rev. 113, 8377–8399 (2013).
Bai, L., Santangelo, T. J. & Wang, M. D. Single-molecule analysis of RNA polymerase transcription. Annu. Rev. Biophys. Biomol. Struct. 35, 343–360 (2006).
Stracy, M. & Kapanidis, A. N. Single-molecule and super-resolution imaging of transcription in living bacteria. Methods 120, 103–114 (2017).
Lerner, E. et al. Toward dynamic structural biology: two decades of single-molecule Förster resonance energy transfer. Science 359, eaan1133 (2018).
Dulin, D., Berghuis, B. A., Depken, M. & Dekker, N. H. Untangling reaction pathways through modern approaches to high-throughput single-molecule force-spectroscopy experiments. Curr. Opin. Struct. Biol. 34, 116–122 (2015).
Juette, M. F. et al. The bright future of single-molecule fluorescence imaging. Curr. Opin. Chem. Biol. 20, 103–111 (2014).
Felce, J. H., Davis, S. J. & Klenerman, D. Single-molecule analysis of G protein-coupled receptor stoichiometry: approaches and limitations. Trends Pharmacol. Sci. 39, 96–108 (2018).
Camunas-Soler, J., Ribezzi-Crivellari, M. & Ritort, F. Elastic properties of nucleic acids by single-molecule force spectroscopy. Annu. Rev. Biophys. 45, 65–84 (2016).
Park, S., Brandani, G. B., Ha, T. & Bowman, G. D. Bi-directional nucleosome sliding by the Chd1 chromatin remodeler integrates intrinsic sequence-dependent and ATP-dependent nucleosome positioning. Nucleic Acids Res. 51, 10326–10343 (2023).
Hoskins, A. A., Gelles, J. & Moore, M. J. New insights into the spliceosome by single molecule fluorescence microscopy. Curr. Opin. Chem. Biol. 15, 864–870 (2011).
Deindl, S. & Zhuang, X. Monitoring conformational dynamics with single-molecule fluorescence energy transfer: applications in nucleosome remodeling. Methods Enzymol. 513, 59–86 (2012).
Tome, J. M. et al. Comprehensive analysis of RNA-protein interactions by high-throughput sequencing-RNA affinity profiling. Nat. Methods 11, 683–688 (2014).
Nutiu, R. et al. Direct measurement of DNA affinity landscapes on a high-throughput sequencing instrument. Nat. Biotechnol. 29, 659–664 (2011).
Jung, C. et al. Massively parallel biophysical analysis of CRISPR-Cas complexes on next generation sequencing chips. Cell 170, 35–47.e13 (2017).
Kleiner, R. E., Dumelin, C. E., Tiu, G. C., Sakurai, K. & Liu, D. R. In vitro selection of a DNA-templated small-molecule library reveals a class of macrocyclic kinase inhibitors. J. Am. Chem. Soc. 132, 11779–11791 (2010).
Basu, A. et al. Measuring DNA mechanics on the genome scale. Nature 589, 462–467 (2021).
Nguyen, U. T. T. et al. Accelerated chromatin biochemistry using DNA-barcoded nucleosome libraries. Nat. Methods 11, 834–840 (2014).
Dann, G. P. et al. ISWI chromatin remodellers sense nucleosome modifications to determine substrate preference. Nature 548, 607–611 (2017).
Severins, I., Joo, C. & van Noort, J. Exploring molecular biology in sequence space: the road to next-generation single-molecule biophysics. Mol. Cell 82, 1788–1805 (2022).
Kinney, J. B. & McCandlish, D. M. Massively parallel assays and quantitative sequence-function relationships. Annu. Rev. Genomics Hum. Genet. 20, 99–127 (2019).
Marklund, E., Ke, Y. & Greenleaf, W. J. High-throughput biochemistry in RNA sequence space: predicting structure and function. Nat. Rev. Genet. 24, 401–414 (2023).
Layton, C. J., McMahon, P. L. & Greenleaf, W. J. Large-scale, quantitative protein assays on a high-throughput DNA sequencing chip. Mol. Cell 73, 1075–1082.e4 (2019).
Boyle, E. A. et al. High-throughput biochemical profiling reveals sequence determinants of dCas9 off-target binding and unbinding. Proc. Natl Acad. Sci. USA 114, 5461–5466 (2017).
Svensen, N., Peersen, O. B. & Jaffrey, S. R. Peptide synthesis on a next-generation DNA sequencing platform. Chembiochem 17, 1628–1635 (2016).
Szymczak, L. C., Kuo, H.-Y. & Mrksich, M. Peptide arrays: development and application. Anal. Chem. 90, 266–282 (2018).
Wu, D. et al. Flow-cell-based technology for massively parallel characterization of base-modified DNA aptamers. Anal. Chem. 95, 2645–2652 (2023).
Li, Z. et al. DNB-based on-chip motif finding: a high-throughput method to profile different types of protein-DNA interactions. Sci. Adv. 6, eabb3350 (2020).
Mamet, N. et al. Ab-initio discovery of tumoricidal oligonucleotides in a DNA sequencing machine. Preprint at bioRxiv https://doi.org/10.1101/630830 (2019).
Buenrostro, J. D. et al. Quantitative analysis of RNA-protein interactions on a massively parallel array reveals biophysical and evolutionary landscapes. Nat. Biotechnol. 32, 562–568 (2014).
Bentley, D. R. et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456, 53–59 (2008).
Aguirre Rivera, J. et al. Massively parallel analysis of single-molecule dynamics on next-generation sequencing chips. Science 385, 892–898 (2024).
Sabantsev, A. et al. Spatiotemporally controlled generation of NTPs for single-molecule studies. Nat. Chem. Biol. 18, 1144–1151 (2022).
Korman, A. et al. Light-controlled twister ribozyme with single-molecule detection resolves RNA function in time and space. Proc. Natl Acad. Sci. USA 117, 12080–12086 (2020).
Brieke, C., Rohrbach, F., Gottschalk, A., Mayer, G. & Heckel, A. Light-controlled tools. Angew. Chem. Int. Ed. Engl. 51, 8446–8476 (2012).
Liu, Y. et al. Very fast CRISPR on demand. Science 368, 1265–1269 (2020).
Hwang, H., Kim, H. & Myong, S. Protein induced fluorescence enhancement as a single molecule assay with short distance sensitivity. Proc. Natl Acad. Sci. USA 108, 7414–7418 (2011).
Kosuri, P., Altheimer, B. D., Dai, M., Yin, P. & Zhuang, X. Rotation tracking of genome-processing enzymes using DNA origami rotors. Nature 572, 136–140 (2019).
Smith, S. B., Finzi, L. & Bustamante, C. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science 258, 1122–1126 (1992).
Strick, T. R., Allemand, J. F., Bensimon, D., Bensimon, A. & Croquette, V. The elasticity of a single supercoiled DNA molecule. Science 271, 1835–1837 (1996).
Cnossen, J. P., Dulin, D. & Dekker, N. H. An optimized software framework for real-time, high-throughput tracking of spherical beads. Rev. Sci. Instrum. 85, 103712 (2014).
Huhle, A. et al. Camera-based three-dimensional real-time particle tracking at kHz rates and Ångström accuracy. Nat. Commun. 6, 5885 (2015).
Andrews, R. et al. Transient DNA binding to gapped DNA substrates links DNA sequence to the single-molecule kinetics of protein-DNA interactions. Preprint at bioRxiv https://doi.org/10.1101/2022.02.27.482175 (2022).
Makasheva, K. et al. Multiplexed single-molecule experiments reveal nucleosome invasion dynamics of the Cas9 genome editor. J. Am. Chem. Soc. 143, 16313–16319 (2021).
Shema, E. et al. Single-molecule decoding of combinatorially modified nucleosomes. Science 352, 717–721 (2016).
Fedyuk, V. et al. Multiplexed, single-molecule, epigenetic analysis of plasma-isolated nucleosomes for cancer diagnostics. Nat. Biotechnol. 41, 212–221 (2023).
Severins, I. et al. Single-molecule structural and kinetic studies across sequence space. Science 385, 898–904 (2024).
Illumina. What Is the Minimum Library Size That Can Be Sequenced? https://knowledge.illumina.com/library-preparation/general/library-preparation-general-faq-list/000006540 (2025).
Nakamura, K. et al. Sequence-specific error profile of Illumina sequencers. Nucleic Acids Res. 39, e90 (2011).
Hamming, R. W. Error detecting and error correcting codes. Bell Syst. Tech. J. 29, 147–160 (1950).
McCann, J. J., Choi, U. B., Zheng, L., Weninger, K. & Bowen, M. E. Optimizing methods to recover absolute FRET efficiency from immobilized single molecules. Biophys. J. 99, 961–970 (2010).
Preus, S., Hildebrandt, L. L. & Birkedal, V. Optimal background estimators in single-molecule FRET microscopy. Biophys. J. 111, 1278–1286 (2016).
Roy, R., Hohng, S. & Ha, T. A practical guide to single-molecule FRET. Nat. Methods 5, 507–516 (2008).
Preus, S., Noer, S. L., Hildebrandt, L. L., Gudnason, D. & Birkedal, V. iSMS: single-molecule FRET microscopy software. Nat. Methods 12, 593–594 (2015).
Thomsen, J. et al. DeepFRET, a software for rapid and automated single-molecule FRET data classification using deep learning. eLife 9, e60404 (2020).
Li, J., Zhang, L., Johnson-Buck, A. & Walter, N. G. Automatic classification and segmentation of single-molecule fluorescence time traces with deep learning. Nat. Commun. 11, 5833 (2020).
Juette, M. F. et al. Single-molecule imaging of non-equilibrium molecular ensembles on the millisecond timescale. Nat. Methods 13, 341–344 (2016).
Wanninger, S. et al. Deep-LASI: deep-learning assisted, single-molecule imaging analysis of multi-color DNA origami structures. Nat. Commun. 14, 6564 (2023).
Götz, M. et al. A blind benchmark of analysis tools to infer kinetic rate constants from single-molecule FRET trajectories. Nat. Commun. 13, 5402 (2022).
Blumhardt, P. et al. Photo-induced depletion of binding sites in DNA-PAINT microscopy. Molecules 23, 3165 (2018).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Edelstein, A., Amodaj, N., Hoover, K., Vale, R. & Stuurman, N. Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. 92, 14.20.1–14.20.17 (2010).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Williams, R. et al. Amplification of complex gene libraries by emulsion PCR. Nat. Methods 3, 545–550 (2006).
Patil, P. V. & Ballou, D. P. The use of protocatechuate dioxygenase for maintaining anaerobic conditions in biochemical experiments. Anal. Biochem. 286, 187–192 (2000).
Aitken, C. E., Marshall, R. A. & Puglisi, J. D. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys. J. 94, 1826–1835 (2008).
Kapanidis, A. N. et al. Alternating-laser excitation of single molecules. Acc. Chem. Res. 38, 523–533 (2005).
Tseng, Q. et al. A new micropatterning method of soft substrates reveals that different tumorigenic signals can promote or reduce cell contraction levels. Lab Chip 11, 2231–2240 (2011).
Aguirre Rivera, J. et al. Massively parallel analysis of single-molecule dynamics on next generation sequencing chips. SciLifeLab Data Repository https://doi.org/10.17044/scilifelab.25705512 (2024).
Panfilov, M. et al. MUSCLE (MUltiplexed Single-molecule Characterization at the Library scalE) protocol data and codes. SciLifeLab Data Repository https://doi.org/10.17044/scilifelab.28008872.v1 (2024).
Sternberg, S. R. Biomedical image processing. Computer 16, 22–34 (1983).
Illumina. What Are the Illumina Sequencing Primer Sequences? https://knowledge.illumina.com/library-preparation/general/library-preparation-general-faq-list/000007129 (2025).
lllumina. What Sequences Do I Use for Adapter Trimming https://knowledge.illumina.com/library-preparation/general/library-preparation-general-reference_material-list/000001314 (2025).
Acknowledgements
We thank M. Lindell (National Genomics Infrastructure, Scilifelab, Uppsala, Sweden) for Illumina sequencing. The original work that led to the development of this protocol was funded by European Research Council (ERC) Advanced Grant ERC-ADG-101092623 (to S.D.), Knut and Alice Wallenberg Foundation grant KAW/WAF 2019.0306 (to S.D.), Knut and Alice Wallenberg Foundation grant KAW 2024.0012 (to S.D.), Cancerfonden grant 22 2106 Pj (to S.D.) and Swedish Research Council project grants VR 03534 and VR 03255 (to S.D.).
Author information
Authors and Affiliations
Contributions
The comprehensive description of the MUSCLE method is based and extends upon the contributions of the authors listed in the initial publication39. S.D. conceived the project, with input from A.S. J.A.R. designed the 3D-printed adapter, built the optical setup with input from A.S. and implemented automated data acquisition. G.M. and J.A.R. conducted MUSCLE experiments. A.S., M.P. and J.G. implemented the trace-registration pipeline. A.S. and M.P. developed the MATLAB pipeline for trace analysis from MUSCLE data. M.P., G.M., A.S. and S.D. wrote the paper, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key reference
Aguirre Rivera, J. et al. Science 385, 892–898 (2024): https://doi.org/10.1126/science.adn5371
Supplementary information
Supplementary Information
Supplementary Figs. 1–5
Supplementary Data
Blueprint for 3D-printing the flow cell adapter
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panfilov, M., Mao, G., Guo, J. et al. Multiplexed single-molecule characterization at the library scale. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01198-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01198-w