Abstract
Accurate differentiation between angina with no obstructive coronary arteries (ANOCA) and mental stress-induced myocardial ischemia (MSIMI) is crucial for tailored treatment strategies, yet public data scarcity hampers understanding. Given the higher incidence of both conditions in women, this study prospectively enrolled 80 female ANOCA and 39 age-matched female controls, subjecting them to three types of mental stress tasks. ECGs were continuously monitored across Rest, Stress, and Recover stages of the mental stress tasks, with PET/CT imaging during the Stress stage to evaluate myocardial perfusion. With PET/CT serving as the gold standard for MSIMI diagnosis, 35 of the 80 ANOCA patients were diagnosed as MSIMI. Using ECG variables from different stages of mental stress tasks, we developed five machine learning models to diagnose MSIMI. The results showed that ECG data from different stages provide valuable information for MSIMI classification. Additionally, the dataset encompassed demographic details, physiological, and blood sample test results of the patients. We anticipate this new dataset will significantly push further progress in ANOCA and MSIMI research.
Similar content being viewed by others
Background & Summary
Angina with no obstructive coronary arteries (ANOCA) is increasingly recognized in contemporary populations especially in women, presenting a relatively higher risk for cardiac events, and posing significant management challenges1,2. Patients with ANOCA commonly endure a high burden of symptoms and may experience repeated presentations to multiple healthcare providers before receiving a diagnosis, which may exacerbate anxiety and depression1. Notably, recent research indicates that women with ANOCA are more susceptible to severe myocardial ischemia during periods of mental stress, known as mental stress-induced myocardial ischemia (MSIMI)3,4,5,6. Singular cardiovascular interventions often prove ineffective for MSIMI, necessitating concurrent psychological interventions to improve patient prognosis7. Therefore, accurate differential diagnosis between ANOCA and MSIMI is crucial for customized treatment and management strategies8.
Despite growing clinical awareness, the diagnosis of these conditions faces significant challenges, especially in resource-intensive primary healthcare settings. This is mainly due to the absence of specific, objective biomarker-based assessments to facilitate differential diagnosis. However, research into ANOCA and MSIMI is hampered by the lack of comprehensive datasets.
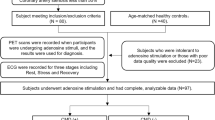
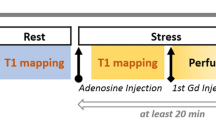
In this study, we prospectively recruited 80 female ANOCA patients and 40 age-matched healthy female controls. Each subject underwent three types of mental stress tasks in the laboratory (including mental arithmetic, public speech describing an event that occurred recently and was emotionally upsetting, and task-modified Stroop test) (Fig. 1). ECG data were continuously recorded throughout the entire experimental procedure, including the Rest, Stress, and Recover stages (Fig. 2) while PET/CT imaging, utilizing a 13N-ammonia tracer, was conducted seven minutes into the stress stage to assess myocardial perfusion. Diagnoses labels derived from ECG (by ST depression ≥0.1 mV) and PET/CT (by summed difference score, SDS ≥ 3) were made by experienced experts following established diagnostic guidelines9 (Fig. 1). PET/CT results were considered the gold standard for MSIMI diagnosis10,11,12,13. Physiological tests and blood samples were collected during the preparation period before mental stress tasks.
Given the clinical accessibility of ECG, we initiated our investigation with an analysis of ECG variables. A thorough review of the literature led to the selection of 88 explainable ECG variables14,15,16, encompassing well-established morphological variables and heart rate variability (HRV) metrics. HRV has been employed to examine the effects of mental stress on the autonomic nervous system17,18,19,20. The entirety of ECG data was divided into three sequential stages: Rest, Stress, and Recover, in accordance with the commencement and termination of mental stress tasks. Employing five conventional machine learning algorithms (Kneighbors, Logistic Regression, Random Forest, SVM, and XGBoost), we developed MSIMI diagnostic models based on ECG variables from various stages. The performances of these models suggested that ECG variables from different stages provided valuable insights for MSIMI classification, and stages such as Rest and Recover should not be disregarded in disease diagnosis. Our dataset further includes data from additional modalities, such as physiological tests and biochemical blood results. We anticipate that this multimodal dataset will significantly propel research forward in ANOCA and MSIMI, especially in addressing the complexities of differential diagnosis.
Methods
Participants
All experiments were conducted at Guangzhou’s Guangdong Provincial People’s Hospital in Guangzhou, Guangdong, China. The study was approved by the Ethics Committee at Guangdong Provincial People’s Hospital (approval ID: GDREC2019298H(R3)).
Participant selection adhered to established criteria:
Criteria for ANOCA inclusion:
-
Women between the ages of 18 and 75 experiencing chest pain or similar symptoms of angina
-
Coronary artery stenosis under 50% as confirmed by coronary CT angiography
Criteria for ANOCA exclusion:
-
Chest pain attributable to non-cardiac circulatory system conditions
-
New York Heart Association’s functional classification stage IV
-
An obstructive myocardial infarction of the coronary artery in the preceding month
-
An instance of apical ballooning syndrome in the preceding month
-
Complications of serious illnesses, such as pulmonary embolism, severe arrhythmias, severe valvular heart disease
-
Complications of severe psychiatric illness such as suicidal behaviors and cognitive impairments
-
Current utilization of postmenopausal hormone treatment or psychotropic medication
-
History of substance misuse, including alcohol or illegal drugs
-
Engagement in other pharmacological studies in the last three months
-
Current pregnancy or lactation
Concurrently, age-matched female control subjects, who do not experience chest pain and are free from coronary artery stenosis, will be sourced through diverse recruitment channels such as WeChat, public notices, online platforms, and radio broadcasts. To confirm the absence of obstructive CAD, control subjects will undergo a CT coronary angiography.
The hospital’s clinical care team identified and preliminarily discussed the study with prospective candidates. Interested individuals were then asked to sign informed consent forms.
Procedures
Patients were hospitalised for the duration of the study. All laboratory tests were conducted between 7:00–10:00 AM while the participants were fasting. This was done to minimize the influence of circadian rhythms and ensure accurate comparison of ECG signals. Blood samples were collected during the preparation period. After a 6-minute supine rest, participants underwent three back-to-back mental stress tasks in a virtual reality setup within the diagnostic PET/CT unit. The tasks were: (1) a Stroop Color and Word Test; (2) a 3-minute speech on a distressing personal event to virtual doctors; and (3) rapidly performing serial subtractions of 7 from a three-digit number. Each task took 4 minutes, cumulating in a 12-minute comprehensive mental stress assessment. At the 7-minute mark of the stress stage, an injection containing 700–900 MBq of 13NH3 was used for myocardial perfusion evaluation and was administered in a “bolus-like” fashion (5 mL of 13NH3), immediately followed by a 10 mL NaCl flush (1 mCi corresponds to 37 MBq) to facilitate PET scanning. Beginning from a restful baseline, ECG monitoring was commenced and extended through six minutes post the mental stress examination, a process illustrated in Fig. 2.
Data acquisition
ECG data
During the mental stress test, continuous ECG monitoring were conducted using the standard 12-lead ECG (Tim Software, Beijing Co., Beijing, China) with a 16-bit precision and a sampling frequency of 500 Hz.
PET/CT data
PET data acquisition and infusion of 13NH3 were commence after the CT scan for PET attenuation correction. All PET/CT examinations were conducted on a single clinical scanner (Biograph HI-REZ 16, Siemens Medical Solution) according to a standardized acquisition protocol, following international guidelines for PET/CT examinations.
Blood collection and tests
Blood samples were obtained on the day of the mental stress study before the mental stress test. The collected blood samples were analysed for cortisol, constituents of the renin-angiotensin-aldosterone system, adrenocorticotropic hormone, and thromboelastography. General and supplemental analyses were conducted to further examine our findings. These data could help to evaluate the pathogenesis of MSIMI from the perspectives of neuroendocrine mechanisms, sex hormone levels, humoral immunity index, and proteome expression.
Psychometric tests
Psychometric tests included the Positive and Negative Affect Schedule (PANAS), Hospital Anxiety and Depression Scale (HADS), Eysenck Personality Scale, Stress Perception Scale (PSS), State-Trait Anxiety Inventory (STAI), Life Event Scale (LES), Post-Traumatic Stress Disorder Checklist—Civilian Version (PCL-C).
Data labeling and processing
ECG data
The diagnosis of MSIMI utilizing ECG criteria was established through consensus among three senior cardiologist, predicated on identifying ST depression exceeding 0.1 mV11. Each cardiologist had 10 or more years of clinical experience, and the final diagnosis was determined based on a consensus agreement among them. The MedEx MECG-200 ECG analysis system was used to filter the signal and characterize the heart’s electrical activity. Moreover, supplementary details were extracted from the hospital’s health record database, including patient’s unique ID, age and the date of data acquisition. The ECG recordings were divided into three parts, namely Rest, Stress, and Recover, based on the start and end times of the mental stress tasks as noted in the experimental records. The recordings were manually split and could be analysed separately or as a whole.
Noise caused by power line interference, baseline wander, and muscle contraction was removed using two median filters (200 ms, 600 ms) and the Daubechies wavelet with a decomposition tree of level 6. After the filtering process, wave peaks were detected, and morphological features and HRV indices were obtained using NeuroKit2, an open-source Python package suitable for both novice and advanced programmers. Information on how to install Python and NeuroKit2 can be found at https://github.com/neuropsychology/NeuroKit.
PET/CT data
PET data was acquired in list-mode and interpreted by two experienced readers following the recommendations of the American Society of Nuclear Cardiology. Prior to interpretation, PET images were screened for errors, such as patient movement, attenuation, reconstruction artifacts, and low count density. Perfusion were calculated using commercially available and previously validated software (QGS + QPS Automatic Quantification, Version 2013.1, Cedars-Sinai Medical Center, Los Angeles, USA). To quantify perfusion, a Summed Difference Score (SDS) was calculated as the difference between the summed stress score and the summed rest score. An SDS of 3 or greater is commonly accepted as indicative of MSIMI (+). Moreover, participants who experienced angina but recorded an SDS below 3 were categorized as MSIMI (−).
Other data
Blood test results, psychometric test results, and other relevant clinical data such as sleep quality and comorbidities were collected along with medical records and recorded in a Case Record Form (CRF) in compliance with Good Clinical Practice requirements. The CRF was used for future analysis.
Data Records
The dataset is available for download from the Science Data Bank, as referenced in citation number21.
Dataset overview
The dataset’s basic information, including dataset description, participant inclusion and exclusion criteria, disease definition, description of the mental stress task procedures, ECG channel descriptions, and diagnostic criteria based on PET/CT and ECG, were stored in separate files in the .json format16. Figure 3 shows the directory tree for our repository and previews of the meta-data.
Dataset description
The ECG data for each participant during each session was saved in a ‘csv’ file format, with two different sampling frequencies available, namely 100 Hz and 500 Hz. The data for each subject (number: 001, 002,…) was stored as a first-level directory. The file naming rules were as follows.
Sub-xxx_baseline-data (or experimental-data or null) datatype (blood-tests, chief-complaint, petct_rest_indicators. et al.) where ‘xxx’ was the subject number (001, 002, …, 025).
The labels, including control, MSIMI (−), and MSIMI (+), are provided in sub-xxx disease.json. For specific diagnostic criteria, please refer to diagnostic-criteria.json (as seen in the PET/CT data section).
Code availability
The code for dataset preparation is not intended to be released as it does not entail any potential for reusability.
Source data
The source data was categorized according to the subject’s recorded stages, i.e., whether during the experiment or not. The baseline data consisted of blood test results, chief complaints, and ECG data recorded at rest before the mental stress tasks. The experimental data included ECG and PET/CT data recorded during the stress stages. The ECG data was the preprocessed raw data saved as ‘.dat’ files named after the subject number and stages. PET/CT data included polar maps, myocardial blood flow (mbf), and summed difference scores (SDS), the three key features for diagnosing MIMI. Among them, mbf is a vital prognosis indicator that has been established by numerous cardiology studies.
Technical Validation
Data quality assessment
To ensure the quality of the ECG recordings, we performed a signal quality assessment by computing established ECG morphology and heart rate variability (HRV) index from the original ECG recordings of the control group under resting conditions, as described in the Methods section. Subsequently, we compared these results with the standard criteria reported in previous studies. (Table 1)
Disease classification
We conducted a detailed literature review to identify 88 ECG-based variables, including ECG morphology and heart rate variability (HRV) variables, which are listed in Table S1. The ECG recordings were extracted and divided into three specific stages according to the mental stress task’s timeline: Rest, Stress, and Recovery. During each stage, the ECG variables were independently calculated, resulting in a total of 264 variables (88 for each stage). Using variables from different stages and their combinations, we developed five classic machine learning models—K-Neighbors, Logistic Regression, Random Forest, SVM, and XGBoost—to distinguish MSIMI from control and ANOCA (diagnostic criteria see in PET/CT data section). The average accuracy of the models across different sets was obtained using leave-one-out cross-validation. The results indicate that ECG data from different stages can provide valuable information for MSIMI classification, and stages like Rest and Recover should not be overlooked during disease diagnosis.
Usage Notes
This dataset has multiple potential uses for mental stress evaluation and daily ischemia detection. The hereby presented dataset and processing tools are provided for public use and may be used with proper citation to the current paper Table 2.
Code availability
For technical validation, we utilized publicly available code without any restrictions. Specifically, we employed the following functions/scripts:
• nk.ecg_peaks.py from the NeuroKit2 package to identify R-peaks in an ECG signal (https://github.com/neuropsychology/NeuroKit).
• find_peaks.py from the SciPy package to identify R-peaks in an ECG signal (https://docs.scipy.org/doc/scipy/reference/generated/scipy.signal.find_peaks.html).
• nk.ecg_delineate.py from the NeuroKit2 package to delineate the QRS complex for morphology features (https://neuropsychology.github.io/NeuroKit/_modules/neurokit2/ecg/ecg_delineate.html#ecg_delineate).
• nk.hrv.py from the NeuroKit2 package to compute Heart Rate Variability (https://neuropsychology.github.io/NeuroKit/_modules/neurokit2/hrv/hrv.html#hrv).
• linear_model.LogisticRegression, svm.SVC, ensemble.RandomForestClassifier, neighbors.KNeighborsClassifier, and XGBClassifier from the Scikit-learn package for classification (https://scikit-learn.org/stable/).
However, we customized and combined these packages to form our own code for the project. The Python program for ECG denoising and feature extraction is publicly available at https://github.com/pengxiaoting1995/MPPD_MSIMI.
References
Samuels, B. A. et al. Comprehensive Management of ANOCA, Part 1—Definition, Patient Population, and Diagnosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol, https://doi.org/10.1016/j.jacc.2023.06.043 (2023).
Belmonte, M. et al. Gender-related differences in absolute coronary flow and microvascular resistance in patients with angina and non-obstructed coronary arteries (ANOCA). Eur. Heart J. 44, ehad655.2138 (2023).
Vaccarino, V. et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom. Med. 76, 171–180 (2014).
Pimple, P. et al. Chest Pain and Mental Stress Induced Myocardial Ischemia: Sex Differences. Am. J. Med. 131, 540–547.e1 (2018).
Vaccarino, V. et al. Association of Mental Stress-Induced Myocardial Ischemia With Cardiovascular Events in Patients With Coronary Heart Disease. JAMA 326, 1818–1828 (2021).
Sullivan, S. et al. Sex Differences in Hemodynamic and Microvascular Mechanisms of Myocardial Ischemia Induced by Mental Stress. Arterioscler. Thromb. Vasc. Biol. 38, 473–480 (2018).
Jiang, W. et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA 309, 2139–2149 (2013).
Practical Approach for Angina and Non-Obstructive Coronary Arteries: A State-of-the-Art Review - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10435829/.
Ma, H. et al. Assessing mental stress on myocardial perfusion and myocardial blood flow in women without obstructive coronary disease: protocol for a mechanistic clinical trial. BMJ Open 10, e038362 (2020).
Zhang, L. et al. A meta-analysis on the prevalence, associated factors and diagnostic methods of mental stress induced myocardial ischemia. J. Transl. Med. 18, 218 (2020).
Stepanovic, J. et al. Mental Stress–Induced Ischemia in Patients With Coronary Artery Disease: Echocardiographic Characteristics and Relation to Exercise-Induced Ischemia. Psychosom. Med. 74, 766 (2012).
Kuroda, T. et al. Effect of mental stress on left ventricular ejection fraction and its relationship to the severity of coronary artery disease. Eur. J. Nucl. Med. 27, 1760–1767 (2000).
Kim, C. K. et al. Detection and reproducibility of mental stress-induced myocardial ischemia with Tc-99m sestamibi SPECT in normal and coronary artery disease populations. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 10, 56–62 (2003).
Zhang, J. et al. Cardiac Electrophysiological Substrate Underlying the ECG Phenotype and Electrogram Abnormalities in Brugada Syndrome Patients. Circulation 131, 1950–1959 (2015).
Singh, I. & Rabkin, S. W. Circadian variation of the QT interval and heart rate variability and their interrelationship. J. Electrocardiol. 65, 18–27 (2021).
Chetran, A. et al. ECG and Biomarker Profile in Patients with Acute Heart Failure: A Pilot Study. Diagn. Basel Switz. 12, 3037 (2022).
Pham, T., Lau, Z. J., Chen, S. H. A. & Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Sensors 21, 3998 (2021).
Shah, A. S. et al. Alterations in heart rate variability are associated with abnormal myocardial perfusion. Int. J. Cardiol. 305, 99–105 (2020).
Costa, M. D., Davis, R. B. & Goldberger, A. L. Heart Rate Fragmentation: A New Approach to the Analysis of Cardiac Interbeat Interval Dynamics. Front. Physiol. 8, 255 (2017).
Kamath, M. V., Watanabe, M. A. & Upton, A. R. M. Heart Rate Variability (HRV) Signal Analysis: Clinical Applications. (Taylor & Francis, Boca Raton, 2013).
Peng, X. & Li, D. Multimodal Physiological and Psychological Database for MSIMI study. ScienceDB https://doi.org/10.57760/sciencedb.07687 (2023).
Acknowledgements
This study was funded by the Young Scientists Fund of National Natural Science Foundation of China (82200558 to D.L.), and the Guangdong Provincial Medical Science and Technology Research Fund Project (A2023027 to D.L.), the National Key Research and Development Program of China (2019YFB1404803 to H.L.), the General Program of National Natural Science Foundation of China (62076076 to H.L.), the Excellent Young Scientists Fund (82122036 to H.L.), and the Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application (2022B1212010011).
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis, writing, Xiaoting Peng and Dantong Li; resources, Huan Ma, Shuxia Wang, Jun Quan; software, Shuai Huang; data curation, Lianting Hu; investigation, supervision, Huiying Liang, Shuxia Wang, Huan Ma, Qingshan Geng.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, X., Li, D., Quan, J. et al. A multimodal physiological and psychological dataset for human with mental stress induced myocardial ischemia. Sci Data 11, 704 (2024). https://doi.org/10.1038/s41597-024-03462-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-024-03462-2
This article is cited by
-
A multimodal dataset for coronary microvascular disease biomarker discovery
Scientific Data (2025)